Gmp Dokumentation
GMP Lab Medicine Tissue BioUltra BioPlus BioMidi BioCompact II BioCompact BioBlood Compare Search Jobs & Careers BIOBASIC The More You Dokumentation Für weitere Dokumente (zB Preisliste, Ersatzteillisten) bitte einloggen Preisliste 21 13 JAN 21 Die BioLineSerie (Broschüre).

Gmp dokumentation. Teste den kostenfreien Demozugang unserer Ausbildung https//wwwhygienebeauftragteronlinede/#demo_reg In diesem Video wird erklärt was ein Reinraum ist u. This Standard Operating Procedure (SOP) can be applied to all pharmaceutical GMPregulated operations such as drug product, active pharmaceutical ingredient, excipient, vaccine and device manufacturing The 6page SOP includes an appendix indicating the locations of pest traps on a site map Regular Price $100 Today $00. And how well do you a.
716 Gmp Documentation Specialist jobs available on Indeedcom Apply to Document Specialist, Senior Document Specialist, Senior Specialist and more!. Du har ikke adgang til denne side For at få adgang kontakt da Eva Madsen ema@pharmakondk. Kursusindholdet gennemgår de lovpligtige træningskrav til ansatte for at kunne udføre processer i et GMPreguleret produktionsmiljø Kurset er tilrettelagt som en kombination af teori, cases og praktisk træning planlægning, opbevaring, håndtering og dokumentation Undervisningen Kurset varetages af seniorkonsulenter fra AlfaNordic.
All our products are manufactured in GMP certified facilities, which means theyre regulated by the FDA to ensure that whats on our labels is whats in our products Our products are the highest quality and purity available anywhere, and we offer a 60day, empty container, moneyback guarantee to prove it!. Und eine FDA und GMP konforme Dokumentation des Rührvorganges zu ermöglichen binderbehaelterbaude binderbehaelterbaude The TMR magnetic stirrer was developed for the pharmaceutical industry and biotechnology to thoroughly mix sensitive products in a sterile and gentle way, and to. Manipu lationssichere Dokumentationsmöglichkeiten (GLP/GMP) durch inte grierten LangzeitRingprotokollspeicher in Kombination mit der standardmässigen Druckerschnittstelle und zusätzlich der seriellen Schnittstelle RS232 für max 8 Schränke (alternativ RS485 für bis zu 16 Schränken), die in Verbindung mit der Software "Celsius 05" (Standard) oder der aufpreispflichtigen FDAkonformen Software "Celsius 05 FDA Edition" nahezu unbegrenzte Programmabläufe thermischer.
WHO defines Good Manufacturing Practices (GMP) as “that part of quality assurance which ensures that products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the marketing authorization” (ref 27) GMP covers all aspects of the manufacturing process defined manu. Eine GMPgerechte Dokumentation von Daten zählt ebenso zu den Lerninhalten wie der Umgang mit Abweichungen und OOS Ergebnissen Das erlangte Wissen wird zusätzlich durch Workshops vertieft Aus dem Inhalt Bedeutung von GMP Regelwerke in Deutschland AMG, AMWHV. The document is information (meaningful data) and its supporting medium, which could be In paper form, CD, Computer Files, Or Microfilm 3 DOCUMENTATION 4 Documentation?.
How familiar are you with record keeping and the required proper documentation?. GMP operations use comprehensive documentation for various purposes in many different ways The GMP system itself is described in work instructions and SOPs Standard Operating Procedures Up to several hundred SOPs exist in a GMP operation and require continuous maintenance. Diverse batch specifikke dokumenter;.
Good manufacturing practices (GMP) are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. Kurset er målrettet akademikere, der gerne vil opnå en grundlæggende viden om GMPkravene i regulerede produktionsmiljøer indenfor life science, og som endnu ikke har et dybt kendskab eller erfaring indenfor området Logistik, planlægning, opbevaring, håndtering og dokumentation Undervisningen Kurset varetages af seniorkonsulenter. The document is information (meaningful data) and its supporting medium, which could be In paper form, CD, Computer Files, Or Microfilm 3 DOCUMENTATION 4 Documentation?.
The management of each operational site is required to define responsibility for origination, distribution, maintenance, change control, and archiving of all GMP documentation and records used within one site’s department or unit Document owners are required to ensure that the documentation and record systems for which they are responsible are specified in the form of detailed SOPs, including all aspects of documentation and records management as described above. GIMP User Manual¶ GIMP comes with a builtin help system Once you have started the program, press F1 for contextsensitive help You may have to install the help. Batch dokumentation, inproceskontrol, stikprøveudtagning, forskrifter, specifikationer, CoA, CoC og GMP certifikater GxPPharma Support A/S udfører også et generelt compliance check af jeres dokumentationssystem, for at afklare evt mangler i forhold til lovgivningen.
GMP Copying Conditions (LGPL) • Introduction to GMP Brief introduction to GNU MP • Installing GMP How to configure and compile the GMP library • GMP Basics What every GMP user should know • Reporting Bugs How to usefully report bugs • Integer Functions Functions for arithmetic on signed integers • Rational Number. GMP steht für "Good Manufacturing Practice" – gute Herstellungspraxis Diese wird durch verschiedene nationale und internationale Vorschriften und Leitlinien geregelt und gewährleistet, dass pharmazeutische Produkte konsistent nach festgelegten Qualitätsstandards hergestellt werden Dokumentation und insbesondere Datenintegrität zu. Note Please note that some of these files are work in progress They're subject to change and may not be of any use because of errors.
GMP Documentation 1 1 GDP 2 cGMP DOCUMENTATION REQUIREMENTS (Good Documentation Practices) 2 3 Document?. Ifølge GMPbekendtgørelsens § 30 har lægemiddelfremstillere pligt til at indføre et effektivt system til behandling af sager vedrørende reklamationer, som gør det muligt at tilbagekalde lægemidler i distributionsnettet omgående og på ethvert tidspunkt. The term GMP refers to Good Manufacturing Practice Regulations These regulations have been implemented by the Food and Drug Administration Authority of United States The regulations were implemented in accordance with the Federal Drug, Food and Cosmetic Act.
Introduction to HACCP Hazard Analysis and Critical Control Point (HACCP) is an internationally recognized system for reducing the risk of safety hazards in food A HACCP System requires that potential hazards are identified and. Unter Validierung bzw Qualifizierung versteht man die Beweisf hrung, dass Verfahren, Prozesse, Ausr stungsgegenst nde, Materialien, Arbeitsg nge oder Systeme tats chlich zu den erwarteten Ergebnissen f hren Betroffen sind alle Unternehmen, die Rohstoffe, Halbfertig oder Fertigprodukte f r medizinische Ger te, Pharmazeutika, Diagnostika, Lebensmittel herstellen Ebenso sind Labore betroffen. GMPAnforderungen an Dokumente Ein papierloses Büro – gibt es das?.
Good Documentation Practice Documentation is a record of our product history GMP documents are legal documents & requirement The “double check” (review) assures that our work is correct All work should be documented at the time work is performed Correct the errors properly Report the deviations if any and implement the CAPA Train all the staffs on GDP and explain the consequences. Dokumente müssen bestimmte Anforderungen erfüllen, um GMPkonform zu sein Neben Gesetzen und Richtlinien, die ebenfalls umfangreiche Anforderungen zur Dokumentation enthalten, hat sich der EUGMPLeitfaden in den letzten Jahren zunehmend als Standard etabliert. If a company is not complying with CGMP regulations, any drug it makes is considered “adulterated” under the law This kind of adulteration means that the drug was not manufactured under conditions.
Is the key to GMP compliance and ensures traceability of. Many people may question the real difference between current Good Manufacturing Process (cGMP) and Good Manufacturing Process (GMP) First off GMP is a set of guidelines, created by the Food and Drug Administration (FDA), that the pharmaceutical industry has put into place to guarantee the products are safe, pure and of great quality. The GDP can be defined as “Good documentation practice is an essential part of the quality assurance and such, related to all aspects of GMP” this definition is based on WHO Clearly written documents prevent errors of various activities in pharma each and every activity is written in specific documents such as SOPs and strictly followed.
Manipu lationssichere Dokumentationsmöglichkeiten (GLP/GMP) durch inte grierten LangzeitRingprotokollspeicher in Kombination mit der standardmässigen Druckerschnittstelle und zusätzlich der seriellen Schnittstelle RS232 für max 8 Schränke (alternativ RS485 für bis zu 16 Schränken), die in Verbindung mit der Software "Celsius 05" (Standard) oder der aufpreispflichtigen FDAkonformen Software "Celsius 05 FDA Edition" nahezu unbegrenzte Programmabläufe thermischer. Proper recordkeeping and an understanding of GMP documentation requirements are vital components of current good manufacturing practice These manufacturing documentation services act as a demonstration of compliance with regulatory requirements Technical Safety Services provides a wide range of cGMP documentation and record keeping services. GMP Engineering Handbuch Projektierungshandbuch Leitfaden zur Durchführung von Automatisierungsprojekten im GMP Umfeld 03/19 A5EAA Einleitung Projektierung im GMP Das zu dieser Dokumentation zugehörige Produkt/System darf nur von für die jeweilige Aufgabenstellung.
Dokumente müssen bestimmte Anforderungen erfüllen, um GMPkonform zu sein Neben Gesetzen und Richtlinien, die ebenfalls umfangreiche Anforderungen zur Dokumentation enthalten, hat sich der EUGMPLeitfaden in den letzten Jahren zunehmend als Standard etabliert. This GMP Documentation training course will suit new, as well as established, Document Controllers, QA and QC personnel and technical personnel involved in generating, approving and using GMP documentation and records The GMP Documentation training course is full of practical tips and advice for having a wellmanaged documentation system will explode some of the myths, remove some of the complexity and refocus upon the rules and guidance and how they are applied. The GMP Documentation and Record Keeping, an Abridged Course, is a shortened course used for demonstration purposes only The full length course poses the questions Can you withstand an FDA audit?.
GMP rules do not allow companies to use draft, trial or outofdate documents in commercial manufacture The instructions, however, should be based on experience with completing the tasks If problems arise, the procedures should be revised to reflect exactly what is required. Teste den kostenfreien Demozugang unserer Ausbildung https//wwwhygienebeauftragteronlinede/#demo_reg In diesem Video wird erklärt was ein Reinraum ist u. GMPAnforderungen an Dokumente Ein papierloses Büro – gibt es das?.
GMP Richtlinien Die Qualifizierung ist der dokumentierte Nachweis, dass die Anlage ihre Anforderung erfüllt https//lehgmbhcom/gmp/qualifizierungvalidi. GMP Richtlinien Die Qualifizierung ist der dokumentierte Nachweis, dass die Anlage ihre Anforderung erfüllt https//lehgmbhcom/gmp/qualifizierungvalidi. The GDP can be defined as “Good documentation practice is an essential part of the quality assurance and such, related to all aspects of GMP” this definition is based on WHO Clearly written documents prevent errors of various activities in pharma each and every activity is written in specific documents such as SOPs and strictly followed.
GMP Documentation 1 1 GDP 2 cGMP DOCUMENTATION REQUIREMENTS (Good Documentation Practices) 2 3 Document?. Anders har sedan 15 år tillbaka inspirerat och utbildat personal inom livsmedelssäkerhet Med humor, kunskap och glädje lär ni er allt ni behöver från denna formidabla föreläsare. 1 Purposes of GMP Documentation There are many different reasons for the creation and maintenance of GMP documentation GMP documents are required for one or more of the following reasons • Keep track of activities • Create legal documents • Provide a historical record • Provide information • Comply with regulations 2.
All our products are manufactured in GMP certified facilities, which means theyre regulated by the FDA to ensure that whats on our labels is whats in our products Our products are the highest quality and purity available anywhere, and we offer a 60day, empty container, moneyback guarantee to prove it!. New laser technology (“unprinters”) under development to do this very thing should raise alarm bells in the GMP world While the cost saving on hardcopy archiving, recycling or secured paper disposal would be significant, the use of this technology would need to be highly controlled and documented What happens to batch record retention?. Here are a few key GMP guidelines that help manufacturers avoid FDA citations and sanctions Document all procedures in manufacturing operations Maintain all records for plant and equipment cleaning processes Mandate techniques for product sampling Ensure approval by QC personnel for all procedures, tests, controls and deviations.
GMP Engineering Handbuch Projektierungshandbuch Leitfaden zur Durchführung von Automatisierungsprojekten im GMP Umfeld 11/18 A5EAA Einleitung Projektierung im GMP Das zu dieser Dokumentation zugehörige Produkt/System darf nur von für die jeweilige Aufgabenstellung. Is the key to GMP compliance and ensures traceability of. Definition of GMP Documentation Definition of GMP Documentation GMP Documentation means all GMP documentation owned by Seller or its Affiliates that relates exclusively to the Product Sample 1.
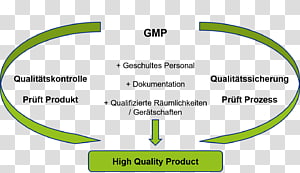
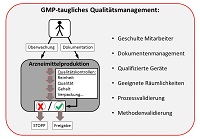
European Commission Choose your language Choisir une. GMPSchulung für Partnerfirmen und Betriebsfremde der Boehringer Ingelheim Pharma GmbH & Co KG Standort Ingelheim Stand • GMPAnforderungen • Personalhygiene • Einschleuseprozedere • Betriebshygiene • GMPgerechte Dokumentation 3 Allgemeine Informationen Die folgende GMPSchulung ist für das laufende Kalenderjahr. The objective of Good Manufacturing Practice (GMP) is to ensure user safety The user being a patient, member of medical staff, members of the community, any one of which, may be negatively impacted by an adulterated, poorly designed or poorly manufactured product The GMP’s when effectively implemented, create a manufacturing environment and management control system where safe, quality, reliable product, is consistently produced from a manufacturing process.
A failure investigation report shall be created in a format that depends on what documentation system is used (paperbased or electronic) All information relating to the deviation must be documented in this report description of the deviation, root causes found and conclusion, results of corrections. GMP Documentation Service We help the firms in designing their documentation system to meet international standards of US FDA, WHO, EU, PICS, TGA, ICH Q7, MHRA The documents shall include QMS, Batch documentation, Validation, Recording forms and formats and SOPs and all relevant /associated GMP documentation. Good manufacturing practices GMP is that part of quality assurance which ensures that products are consistently produced and controlled to the quality standards appropriate to their intended use GMP is aimed primarily at diminishing the risk inherent in any pharmaceutical production.

Gmp Basistraining Gmp Navigator

Gmp Upgrade Calibration Qualification Validation Testotis Com

Kernprozesse Im Pharmalabor Gmp Konforme Analytik Von Der Probenahme Bis Zur Dokumentation Pdf Download
Gmp Dokumentation のギャラリー

Comparison Of Eu Gmp Guidelines With Who Guidelines Identification Of The Cost Intensive Requirements Pdf Free Download
Www Waters Com Webassets Cms Library Docs Local Seminar Presentations Ge Events Ge 17 separation days Dortmund 1 data integrity managing di and cc 169 Pdf

Fallstudie Lessonia Modernisiert Gmp Dokumentation M Files
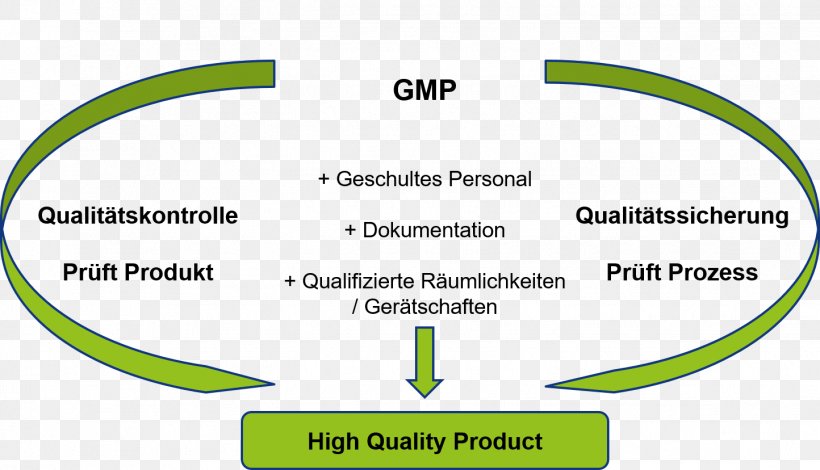
Good Manufacturing Practice Organization Ticeba Gmbh Rheacell Gmbh Co Kg Technical Standard Png 1444x8px Good

Gmp Gerechte Dokumentation In Der Pharmaindustrie Gmp Verlag Peither Ag Pressemitteilung Pressebox
2

Sitemap Xml Elastic Co Elastic Co Elastic Co

Gmp Compliant Calibration For The Life Sciences Industry Endress Hauser

Perspektiven Life Sciences 19 Endress Hauser Ag Kiosk

Chemielaborant In Qc 100 M W D At Unisite Ag Grabjobs
2

Ppt Wp 4 Gmp Faciliteter For Bioterapi Swecrin Temamote Stockholm 1 Oct 08 Pontus Blomberg Karolinska Universitetssjukhu Powerpoint Presentation Id

Annex 1 Conference European Compliance Academy

Advertising And Analysis Platform For Intelligent Online Marketing Diva E
Iso And Gmp Compliant Quality Management With Integrated Administration Of Qualifications Representation Of The Requirements Of The Dakks And Fda For Quality Management

Papierbasierte Und Elektronische Dokumentation Im Pharmaunternehmen Von Michael Dr Hiob Markus Dr Limberger Markus Roemer Markus Prof Dr Veit Cornelia Wawretschek Faltershop At

Harrow Flash Baby 2 In 1 髮 Shower Gel Baby Wash Baby Wash 髮 50ml X 5 Bottles
2

Ppt Wp 4 Gmp Faciliteter For Bioterapi Swecrin Temamote Stockholm 1 Oct 08 Pontus Blomberg Karolinska Universitetssjukhu Powerpoint Presentation Id

Gmp Kursus

Gmp Konforme Iq Oq Dokumentation Testo Se Co Kgaa

Perspektiven Life Science Endress Hauser Ag Kiosk

Was Ist Gmp
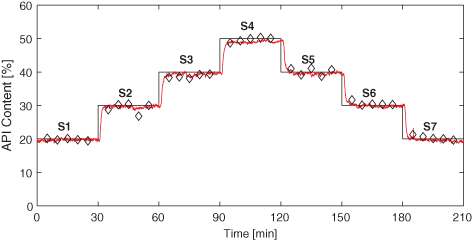
Hot Melt Extrusion As A Continuous Pharmaceutical Manufacturing Process Springerlink

Gmp International Gmp International

Comparison Eu Vs Who Guidelines

Comparison Eu Vs Who Guidelines

Gmp Anforderungen An Dokumente

Gmp Verlag Peither Ag Gmp Publishing Peither Gmp Tea Die Zweite Episode Ist Da Wo Muss Ich Dokumentieren Wer Darf Dokumente Erstellen Das Und Noch Viel Mehr Erfahren

Gmp Fda Gerechte Dokumentation In Der Herstellung D 1 Gmp Navigator

Ppt Wp 4 Gmp Faciliteter For Bioterapi Swecrin Temamote Stockholm 1 Oct 08 Pontus Blomberg Karolinska Universitetssjukhu Powerpoint Presentation Id

Gmps In Storage Transportation And Cold Chain Gmp Navigator
Qualifizierung Cenguru

Artwork For Pharma Training And Communications Michael Cucurullo
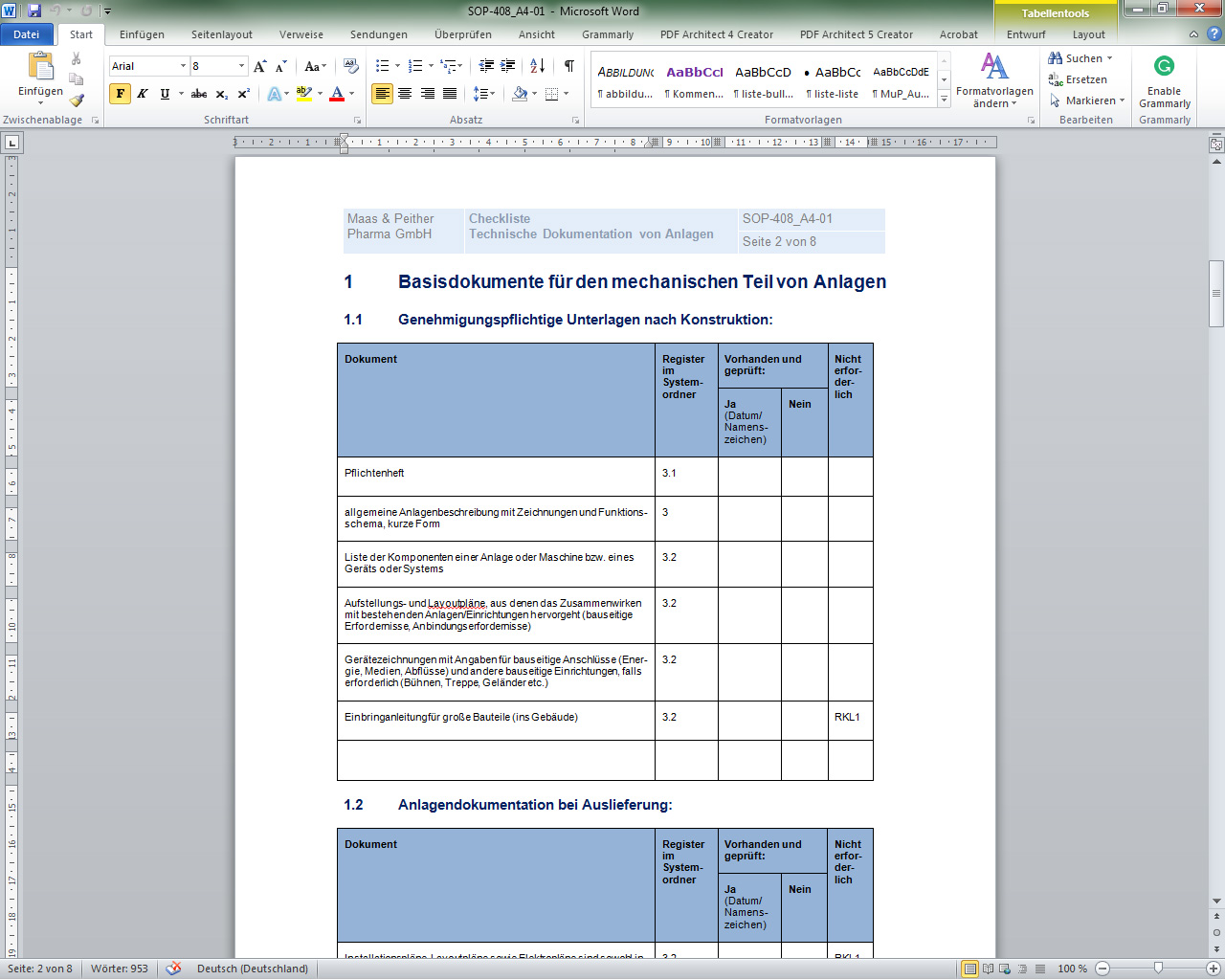
Sop Technische Dokumentation Pharmabetrieb Gmp Verlag Peither Ag

Gmp Gerechte Dokumentation In Der Pharmatechnik Pt 1 Gmp Navigator

Guide For Gmp Documentation And Records

Gxp Konforme Dokumentation Leicht Gemacht Omnilab Blog

Comparison Of Eu Gmp Guidelines With Who Guidelines Identification Of The Cost Intensive Requirements Pdf Free Download

Comparison Of Eu Gmp Guidelines With Who Guidelines Identification Of The Cost Intensive Requirements Pdf Free Download
Duran Duran Pure
Ticeba Transparent Background Png Cliparts Free Download Hiclipart

Iso And Gmp Compliant Quality Management With Integrated Administration Of Qualifications Representation Of The Requirements Of The Dakks And Fda For Quality Management

Good Manufacturing Practice Organization Ticeba Gmbh Rheacell Gmbh Co Kg Technical Standard Gmp Angle Text Png Pngegg

Sterilizers For The Pharmaceutical Industry Individual Belimed Inc
Was Ist Gmp

Pharma Ingenieur Ingenieur Pharmatechnik Als Experte Gmp Dokumentation M W In Wuppertal Elberfeld Gesucht Von Bayer Ag

Cato Gmp And Documentation

Gmp Schulung Fur Partnerfirmen Der Boehringer Ingelheim Pharma Gmbh Co Kg Div Launch Production Site Germany Stand Ppt Video Online Herunterladen

Good Manufacturing Practices Gmp Weltweite Richtlinien Zur Herstellung Von Arzneimitteln German Edition Zeilhofer Ficker I Amazon Com Books
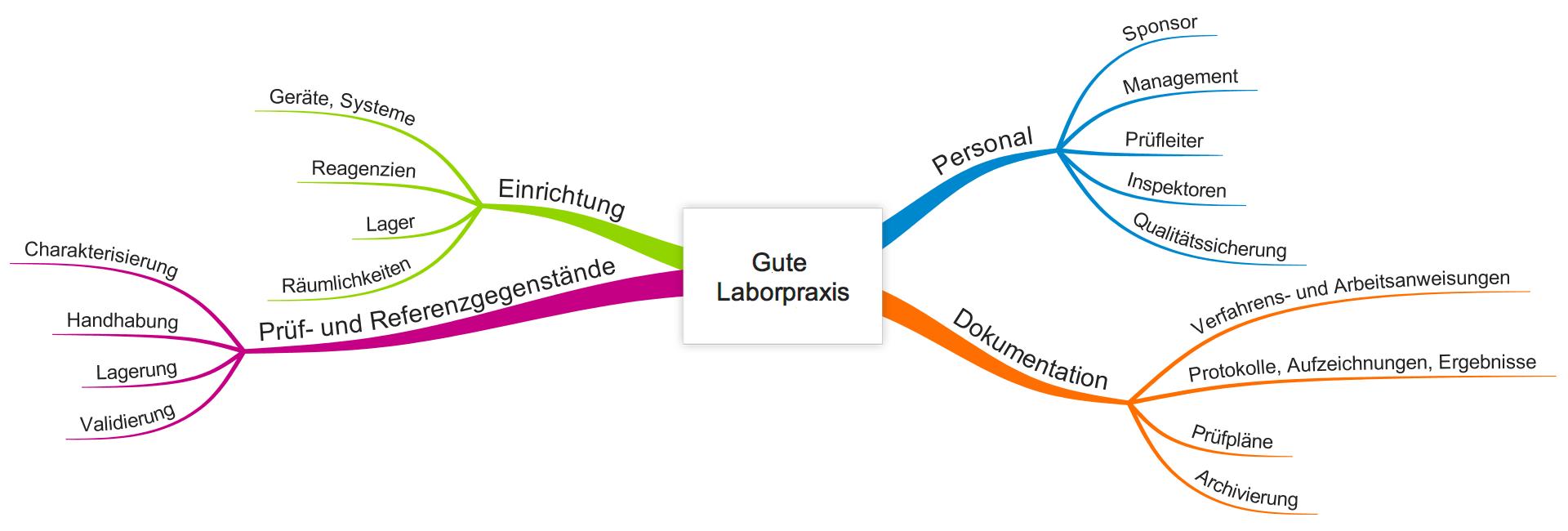
Good Laboratory Practice Glp Gute Laborpraxis

Good Manufacturing Practice Text Png Download 500 500 Free Transparent Good Manufacturing Practice Png Download Cleanpng Kisspng

Good Manufacturing Practice Eu Leitfaden Der Guten Herstellungspraxis Fur Arzneimittel Und Wirkstoffe Mit Arzneimittel Und Wirkstoffherstellungsverordnung Cosmetics Text European Union Medicinal Materials Transparent Background Png Clipart Hiclipart
Www Bundesgesundheitsministerium De Fileadmin Dateien 3 Downloads Statistiken Gkv Bekanntmachungen Gmp Leitfaden Anlage 1 Zur Bekanntmachung Kapitel 4 Dokumentation Pdf

Sitemap Xml Elastic Co Elastic Co Elastic Co

Dokumentation Papierbasiert Elektronisch Pharmaunternehmen Gmp Verlag Peither Ag

Abkurzungen Good Manufacturing Practice 8 Gs1 Organisation Zur Vergabe Und Internationalen Standardisie Rung Pdf Document

Robotec Solutions Ag Documentation Gmp

Manufacturing Associate Mitarbeiter Produktion Gmp Pilot Plant M W D At Bristol Myers Squibb In Calle Munich Montes Olimpicos Tijuana Mexico R 1

Dokumentation By Walter Bernd On Prezi Next
D Nb Info 34

High Speed Door Fire Cleanroom Good Manufacturing Practice Lighting Fire Door Transparent Png

Certocontrol Posts Facebook

Comparison Eu Vs Who Guidelines
Http Www Swisscleanroomconcept Ch Userfiles File Ecv leseproben Eu Guide To Good Manufacturing Pdf

Comparison Eu Vs Who Guidelines
Http Www Pharma Gally Ch Pdf Risikoanalyse Anlageplaner Pdf
Www Phenomenex Com Info Webdocumentserve De 1 8 Pdf

Gmp Dokumentation Warum Weniger Papier Auch Weniger Probleme Bedeutet

Live Online Training Setting Specifications And Acceptance Criteriaim Auftrag Der Eca Academy Gmp Navigator
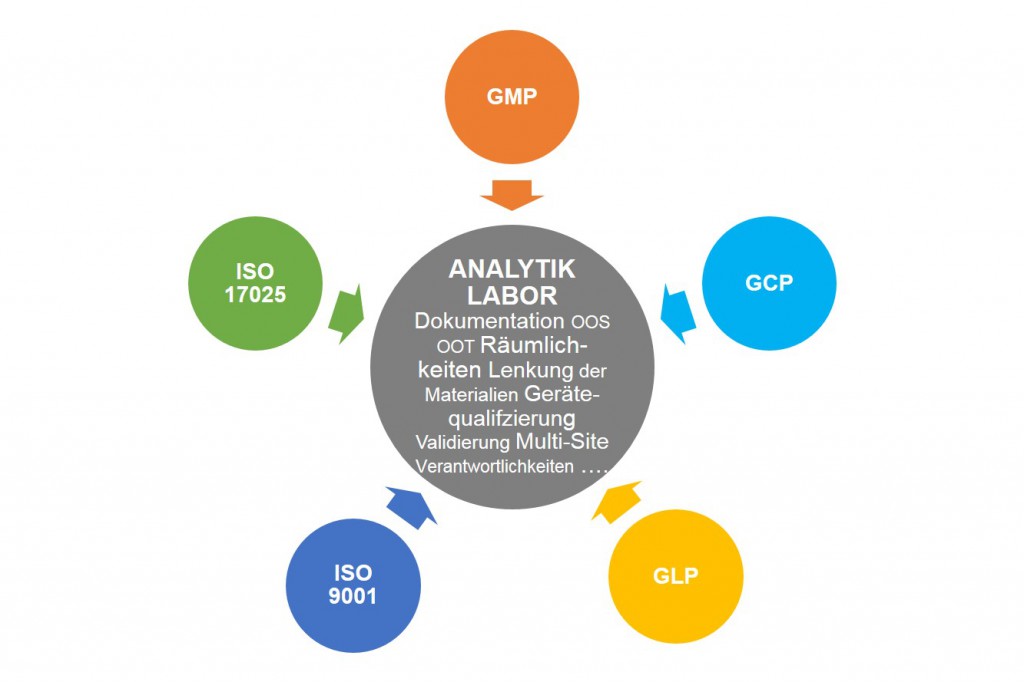
Gute Analytiklabor Praxis Gmplan Gmbhgmplan Gmbh

Calameo Cis Gmp News 19 04 Autumn

Comparison Of Eu Gmp Guidelines With Who Guidelines Identification Of The Cost Intensive Requirements Pdf Free Download

Aqu Service Services Bwt

Cnc International Qualitat
Azslide Com Download Umsetzung Gmp Normen Fr Herstellung 5a38fbbc1723ddf Html

Engel Gmp Dokumentation Engel Global

Demo Gmp Elearning

Good Manufacturing Practices Gmp Weltweite Richtlinien Zur Herstellung Von Arzneimitteln German Edition Zeilhofer Ficker I Amazon Com Books

Bsv Bioscience Gmbh Posts Facebook

Ppt Wp 4 Gmp Faciliteter For Bioterapi Swecrin Temamote Stockholm 1 Oct 08 Pontus Blomberg Karolinska Universitetssjukhu Powerpoint Presentation Id

Creating Operator Input Alarms Gmp Engineering Manual Simatic Wincc Tia Portal Id Industry Support Siemens

Quality By Design Process Analytical Technology Gmp And Regulatory Affairs Request Pdf
Www Sgs Com Media Global Documents Technical Documents Technical Bulletins Sgs Lss Capa Management In A Gmp Environment En 14 Pdf

Gmp Schulung Fur Partnerfirmen Und Betriebsfremde Der Transfer Auf Eine Fur Den Gmp Bereich
Www Transfusionguidelines Org Document Library Documents Assessment Questionnaire Answers Download File Oig Tools Qa Gmp Answers For Hospitals Pdf
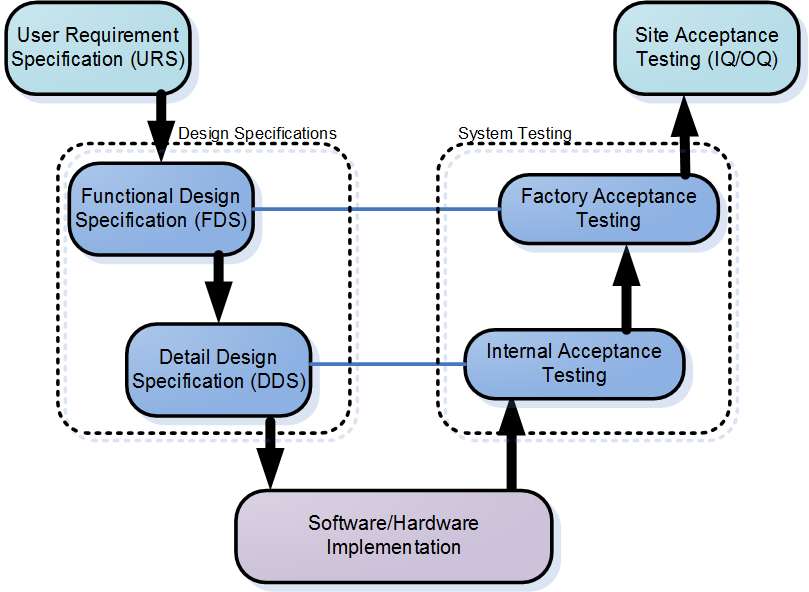
Gamp5 Urs Fds Hds Sds Ts Quality Engineering
Enabling Gmp Compliant Configuration Wincc Advanced V13 0 Sp1 Id Industry Support Siemens
Pdf4pro Com File 6acdf Wp Content Uploads Mpa Pdf Pdf
2
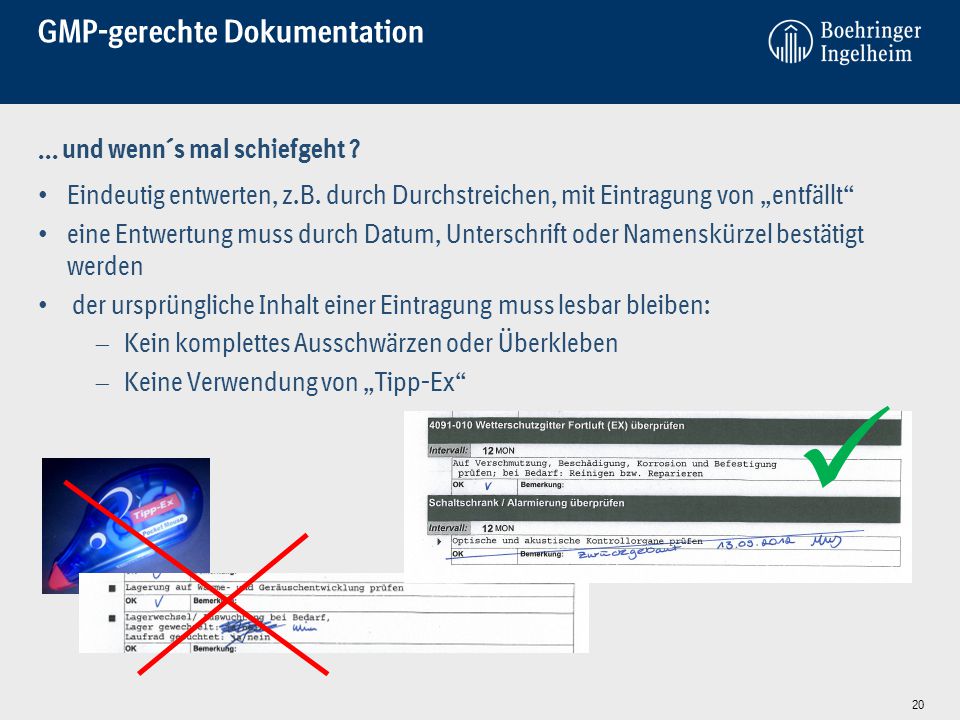
Gmp Schulung Fur Partnerfirmen Der Boehringer Ingelheim Pharma Gmbh Co Kg Div Launch Production Site Germany Stand Ppt Video Online Herunterladen
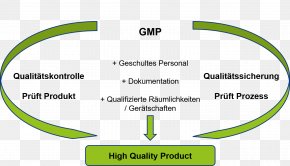
Project Science Metrology Organization Good Manufacturing Practice Png 800x800px Project Chin Computer Cluster Face Forehead Download Free
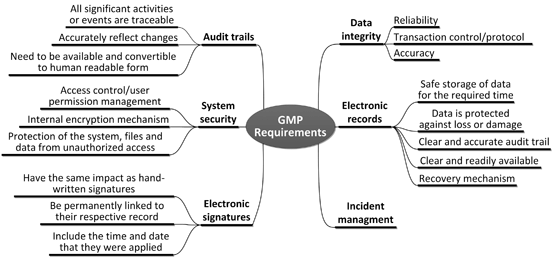
Hot Melt Extrusion As A Continuous Pharmaceutical Manufacturing Process Springerlink

Comparison Of Eu Gmp Guidelines With Who Guidelines Identification Of The Cost Intensive Requirements Pdf Free Download
2

Neuerscheinung Des Gmp Verlags Gmp Trainer Schulungspaket 3 Dokumentation Gmp Verlag Peither Ag Pressemitteilung Pressebox

Gmp Fda Gerechte Validierung Regulatorische Anforderungen Dokumentation Qualifizierung Von Alt Und Neuanlagen Prozess Und Reinigungsvalidierung Computergestutzte Systeme Abebooks



