In Vitro Testing
Jan , 21 (The Expresswire) Global “InVitro Toxicology Testing Market” forecast report delivers important insights and provides a complete analysis of.
In vitro testing. Invitro testing removes a small bit of tissue from a living body and tests the tissue itself, leaving the living organism safe and sound The Latin term in vitro means “in glass” and refers to the tissues being tested in glass test tubes Invitro testing is one of the many alternative options to avoid animal testing. DUBLIN, Jan , 21 /PRNewswire/ The "Demand for HighVolume Testing Driving the Growth of the US Clinical Chemistry and Immunoassay In Vitro Diagnostics Market, " report has been added. In vitro testing for the assessment of your chemical is increasingly important as animal use continues to be replaced, refined and reduced Covance is helping lead innovation in this area so by partnering with us you will benefit from extensive experience in standard techniques and novel solutions.
Jan , 21 (The Expresswire) Global “InVitro Toxicology Testing Market” forecast report delivers important insights and provides a complete analysis of. DUBLIN, Jan , 21 /PRNewswire/ The "Demand for HighVolume Testing Driving the Growth of the US Clinical Chemistry and Immunoassay In Vitro Diagnostics Market, " report has been added. DUBLIN, Jan , 21 /PRNewswire/ The "Demand for HighVolume Testing Driving the Growth of the US Clinical Chemistry and Immunoassay In Vitro Diagnostics Market, " report has been added.
It is the experiment or observations done on the tissue outside of the living organism in a controlled environment, usually using Petri dishes and test tubes Most experiments in cellular biology are done through in vitro studies and are not conducted in the organism’s natural environment or inside a living organism. In vitro is used to describe work that’s performed outside of a living organism This can include studying cells in culture or methods of testing the antibiotic sensitivity of bacteria. The new broad usage test is the first in vitro diagnostic product being developed by IDT, which has long been an industry leader in the manufacture of highquality custom nucleic acids and serves over 130,000 life sciences researchers in more than 100 countries The PrimeTime SARSCoV2/Flu Test combines a number of IDT’s components into a single test solution, which will be especially.
In vitro diagnostics are tests done on samples such as blood or tissue that have been taken from the human body. In vitro testing is performed outside of the whole living organism as part of proof of concept (POC) Utilizing tissue samples, isolated organs and cell cultures, explants, cell lines, and even subcellular fractions, in vitro testing allows for fast, repeatable, controllable tests. Overview Health care providers rely on a variety of tools to diagnose conditions and guide treatment decisions Among the most common and widely used are in vitro diagnostics (IVDs), which are clinical tests that analyze samples taken from the human body Patients may receive—or forgo—medical care based on diagnostic test results, making it critically important that tests are reliable.
Reliable in vitro tests can offer the potential to improve a diagnosis of DHR and influence medical decision making Importantly, in vitro testing is frequently not performed as a test in isolation but rather as a component of a diagnostic algorithm along with additional tests. In Vitro Assays In vitro assays for the detection of adventitious virus are required when using mammalian cell lines for the production of biopharmaceutical and biotechnology products The ICH Guidelines (Section 322 from the EMA version of ICH Q5A) requires that samples from the master cell bank, end of production cell bank and bulk harvest be inoculated into cell cultures susceptible to human and animal viruses. InVitro Testing Laboratory Scientists in our InVitro testing labs are well trained on ASTM, AATCC, AOAC, CLSI and EN standard methods The efficacy testing services of this laboratory include MIC/MBC, biofilm prevention and removal, timekill kinetics, cleanroom disinfectant validation, the zone of inhibition and custom efficacy studies for disinfectants, antimicrobials and other products requiring EPA registrations and FDA 510 (k) submissions.
Ortho Clinical Diagnostics is hoping to pay down a large portion of its debts by riding a wave of COVID19 and broader in vitro lab testing demands to a $15 billion IPO The company is offering. In the cosmetics industry, invitro testing is used to confirm the lack of certain toxic properties in cosmetic and personal care products, as well as their ingredients It can be used both to test the efficacy of products and to achieve regulatory approval For instance, data on skin irritation effects are required by the following legislation. The global invitro toxicology testing market is expected to reach USD 1042 Billion by 25, from USD 63 Billion in 17 growing at a CAGR of 65% during the forecast period of 18 to 25 The upcoming market report contains data for historic years 17, the base year of calculation is 17 and the forecast period is 18 to 25.
In vitro allergy testing determines whether a patient’s serum contains immunoglobulin E (IgE) antibodies against specific allergens of clinical importance The radioallergosorbent (RAST) test was developed for in vitro (blood) measurement of specific IgE in a patient’s serum. In vitro comes from the Latin term "in glass" The term refers to studies of biological properties that are done in a test tube (ie in a glass vessel) rather than in a human or animal In vitro studies are often contrasted to in vivo ("in life") studies which are done inside an organism. Invitro toxicology testing is defined as the cultured bacteria being affected by the chemical substances These methods are carried to identify dangerous chemicals to confirm the lack of assured contaminated properties in the initial stages of the growth of new substances which include food additives, therapeutic drugs, and agricultural chemicals.
The key distinction between FDAreviewed IVDs and LDTs is where they are made LDTs are designed and used in a single laboratory, and are sometimes referred to as “inhouse” tests 5 LDTs are developed in facilities ranging from physicians’ offices, hospitals, and academic medical centers to large testing companies 6 Though LDTs may contain the same or similar components as FDAreviewed tests, they must be developed and used within the same facility FDA has historically viewed LDTs. An in vitro test method in which a test substance is applied to a semipermeable membrane Damage to macromolecules in the membrane is measured to assess the test substance’s potential to cause eye irritation. InVitro Dermatological Testing Invitro safety dermatological testing is recommended prior to human clinical trials to evaluate product safety Product manufacturers are responsible for determining personal care product safety and efficacy Safety assessment experts can help determine if appropriate and sufficient data on proposed formulations is available or if additional toxicological evaluations need to be conducted.
Early developments There was a transient biochemical pregnancy reported by Australian Foxton School researchers in 1953 John Rock was the first to extract an intact fertilized egg In 1959, Min Chueh Chang at the Worcester Foundation, proved fertilisation in vitro was capable of proceeding to a birth of a live rabbitChang's discovery was seminal, as it clearly demonstrated that oocytes. In vitro testing is performed outside of the whole living organism as part of proof of concept (POC) Utilizing tissue samples, isolated organs and cell cultures, explants, cell lines, and even subcellular fractions, in vitro testing allows for fast, repeatable, controllable tests These cells or tissues are grown in a lab environment and used to study effects without the need of living subjects. This latest report studies Invitro Colorectal Cancer Screening Tests market research report is replete with precise analysis from radical studies, specifically on queries that approach Market size, trends, share, forecast, outlook, production, and futuristic developments trends and present and future market status The Invitro Colorectal Cancer Screening Tests market report focuses on.
In Vitro Toxicology Testing An overview of In Vitro Toxicology Testing assays using cellbased tests to 3D tissue models In Vitro Toxicology Experts In VitroToxicology Testing Effective & Affordable NonAnimal In VitroToxicity Testing Alternatives In VitroSensitization. In vitro testing has come a long way in the past several years The cosmetic industry has benefited from advances in this form of testing, especially in regard to assessing the sensitivity of cosmetic ingredients One of the newest forms of in vitro testing that the cosmetic industry is benefiting from involves 3D reconstruction of human skin models. This latest report studies Invitro Colorectal Cancer Screening Tests market research report is replete with precise analysis from radical studies, specifically on queries that approach Market size, trends, share, forecast, outlook, production, and futuristic developments trends and present and future market status The Invitro Colorectal Cancer Screening Tests market report focuses on.
It is the experiment or observations done on the tissue outside of the living organism in a controlled environment, usually using Petri dishes and test tubes Most experiments in cellular biology are done through in vitro studies and are not conducted in the organism’s natural environment or inside a living organism This results in the limited success of the experiments in simulating the actual conditions inside an organism and makes its outcome less precise. In vitro studies are performed with microorganisms, cells, or biological molecules outside their normal biological context Colloquially called "testtube experiments", these studies in biology and its subdisciplines are traditionally done in labware such as test tubes, flasks, Petri dishes, and microtiter plates Studies conducted using components of an organism that have been isolated from their usual biological surroundings permit a more detailed or more convenient analysis than can be done w. This latest report studies Invitro Colorectal Cancer Screening Tests market research report is replete with precise analysis from radical studies, specifically on queries that approach Market size, trends, share, forecast, outlook, production, and futuristic developments trends and present and future market status The Invitro Colorectal Cancer Screening Tests market report focuses on.
Invitro tests for the longterm safety evaluation of drugs offer certain advantages Specific properties of drugs can be identified including mutagenic and carcinogenic effects The mechanisms leading to toxicity can be assessed Tissue from several species, including man, can be examined. These alternatives to animal testing include sophisticated tests using human cells and tissues (also known as in vitro methods), advanced computermodeling techniques (often referred to as in silico models), and studies with human volunteers These and other nonanimal methods are not hindered by species differences that make applying animal test results to humans difficult or impossible, and they usually take less time and money to complete. InVitro Testing Laboratory Scientists in our InVitro testing labs are well trained on ASTM, AATCC, AOAC, CLSI and EN standard methods The efficacy testing services of this laboratory include MIC/MBC, biofilm prevention and removal, timekill kinetics, cleanroom disinfectant validation, the zone of inhibition and custom efficacy studies for disinfectants, antimicrobials and other products requiring EPA registrations and FDA 510 (k) submissions.
In Vitro Release Testing (IVRT) Measurement of drug release from complex dosage forms intended for ophthalmic and topical applications is fundamental to drug product development and product equivalence testing. Is In Vitro a Good Alternative to Animal Testing for Efficacy Testing of Cosmetics Before a new cosmetic product enters the market, it must be subjected to a series of tests, from safety and toxicity testing to efficacy testing and claim substantiation In the past, it was common to use animals for such tests. In vitro testing for the assessment of your chemical is increasingly important as animal use continues to be replaced, refined and reduced Covance is helping lead innovation in this area so by partnering with us you will benefit from extensive experience in standard techniques and novel solutions.
An array of in vitro testing methods can be used to verify that cosmetic products and ingredients are free from components that are cytotoxic, mutagenic, or capable of causing skin and eye irritation (temporary damage) or corrosion (permanent damage). In vitro tests include studies of cosmetics materials’ cytotoxicity, evaluation of the level of synthesis and cell activity of enzymatic proteins, effects of growth agents and various types of compounds ultimately influencing the skin aging processes. In vitro sensitization assay testing according to OECD guidelines and GLP standards Our laboratories historically specialize in in vitro studies We offer a portfolio of assays ranging from the wellestablished to the most cuttingedge, guided by in silico modelling.
In Vitro is basically anything that is being experimented or tested on outside of the living organism in a controlled laboratory setting When most people think about in vitro, they think of the conception method, in vitro fertilization But what many people do not know is that it can be used as an alternative for animal testing. In vitro refers to a phenomenon in which a given procedure is performed in a controlled environment outside of a living organism The majority of cellular experiments are performed in vitro as it is less expensive But, the regeneration of the physiological conditions of an organism is difficult inside a test tube. A test performed in vitro ("in the glass") means that it is done outside of a living organism and it usually involves isolated tissues, organs or cells You can use in vitro data to fully or partly fulfil information requirements that would otherwise need data to be generated with tests on living organisms ( in vivo tests).
In vitro studies include drugdrug interaction, BCS biowaivers derived from Caco2 testing, and ADME screening assays;. Before submitting an IND application, the concerned drug must go through a comprehensive series of invitro and invivo toxicity testing to ensure maximum safety in clinical trials Considering the ethical issues and the cost of invivo animal tests, the pharmaceutical industry now relies more on invitro methods for toxicity testing in the drug development phase. This latest report studies Invitro Colorectal Cancer Screening Tests market research report is replete with precise analysis from radical studies, specifically on queries that approach Market size, trends, share, forecast, outlook, production, and futuristic developments trends and present and future market status The Invitro Colorectal Cancer Screening Tests market report focuses on.
Company developed CellPort Technologies, a suite of preclinical in vitro tests for interactions between drugs and specific transport proteins identified by FDA as key mediators of drug safety. DUBLIN, Jan , 21 /PRNewswire/ The "Demand for HighVolume Testing Driving the Growth of the US Clinical Chemistry and Immunoassay In Vitro Diagnostics Market, " report has been added. InVitro Efficacy Testing BioScience Laboratories has twenty three years of experience in conducting clinical & invitro studies on antimicrobial products used in food service, healthcare, and janitorial sanitation settings, as well as antimicrobial products used by the general consumer.
Invitro Toxicity Testing in Drug Development Posted by krishna kumar Before submitting an IND application, the concerned drug must go through a comprehensive series of invitro and invivo toxicity testing to ensure maximum safety in clinical trials. We offer in vitro testing services to a variety of industries, including cosmetics/personal care, household products, specialty chemicals, consumer products and pharmaceuticals READ ABOUT OUR INDUSTRY WORK. In vitro diagnostic (IVD) devices are tests performed on samples taken from the human body, such as swabs of mucus from inside the nose or back of the throat, or blood taken from a vein or.
ONdrugDelivery Magazine, Issue 65 (Mar 16), pp 2630 Tony Copley examines the regulatory framework for transdermal and topical products and popular, effective and costefficient in vitro testing techniques for quantifying their release characteristics Whether for topical action, or as a means of introducing systemically acting drugs into the body, the skin is an important route of administration for a wide range of pharmaceutical products. Absorption Systems, LP (Exton, Pennsylvania, US) preclinical contract research organization;. The In Vitro Toxicology Testing Market report is a collection of pragmatic information, quantitative and qualitative estimation by industry experts The report also provides the qualitative results of diverse market factors on its geographies and segments The report provides an insight into the market dynamics and various trends and opportunities associated with the global In Vitro Toxicology Testing market.
Studies are usually done in vitro first for ethical reasons In vitro studies allow a substance to be studied safely, without subjecting humans or animals to the possible side effects or toxicity of a new drug Researchers learn as much as possible about a drug before exposing humans to potential negative effects. In vitro testing has come a long way in the past several years The cosmetic industry has benefited from advances in this form of testing, especially in regard to assessing the sensitivity of cosmetic ingredients One of the newest forms of in vitro testing that the cosmetic industry is benefiting from involves 3D reconstruction of human skin models. In Vitro Toxicology Laboratory Testing Services & Consulting Enthalpy provides in vitro toxicology services for tobacco, smoke and eVapor With a robust understanding of tobacco products and toxicological sciences, we are able to study early and late genotoxic and cytotoxic effects of individual compounds and various materials (ie, plant, animal and human tissues, agrochemicals, drugs, personal care products, and natural products).
Jan , 21 (The Expresswire) Global “InVitro Toxicology Testing Market” forecast report delivers important insights and provides a complete analysis of. Jan , 21 (The Expresswire) Global “InVitro Toxicology Testing Market” forecast report delivers important insights and provides a complete analysis of. Jan , 21 (The Expresswire) Global “InVitro Toxicology Testing Market” forecast report delivers important insights and provides a complete analysis of.
Many experiments in cellular biology are conducted outside of organisms or cells One of the abiding weaknesses of in vitro experiments is that they fail to replicate the precise cellular conditions of an organism, particularly a microbe.

In Vitro Testing Services
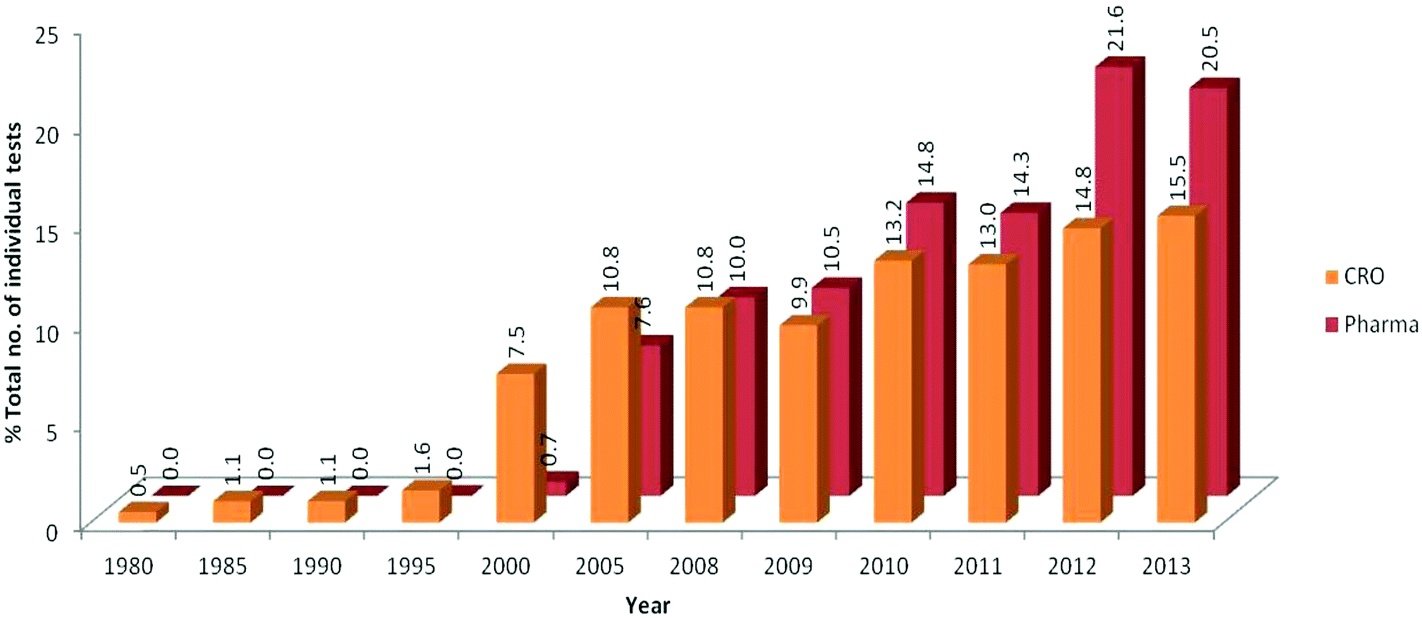
Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd

What Are In Vitro Assays With Pictures
In Vitro Testing のギャラリー

Better Animal Testing Alternatives Are Coming To U S Mddionline Com

Saving The Animals New Ways To Test Products The New York Times

Cellular In Vitro Testing Methods And Protocols 1st Edition John

In Vitro Testing Services Qacs The Challenge Test Lab
Pre Clinical Testing With In Vitro Model May Streamline Vaccine Development

Advinus Now Offers In Vitro Testing For Skin Irritation And Corrosion Episkin Tests Eurofins Advinus

A New Paradigm For In Vitro Toxicology Tests Pharma World

In Vitro The Remarkable Rise Of Animal Alternatives Understanding Animal Research Understanding Animal Research

In Vitro Non Animal Testing Using The Reconstructed Human Epidermis Model Cyprotex

Developing In Vitro Diagnostic Tests The Biomedical Scientist Magazine Of The Ibms

In Vitro Testing In China A Brief Overview Invitrointl

In Vitro Permeation Testing Ivpt Absorption Systems
Antimicrobial Development In Vitro Testing Laboratory Specialists Inc

Biomaterial In Vitro Testing 2 0 Standardized Quantitative Resource Saving An Innovative In Vitro Test System For Quantitative Testing Methods In Direct Cell Material Contact

Laboratories Abich Ca Biological And Chemical Analysis

Global In Vitro Toxicity Testing Market To Reach 8 8bn By 23

Drug Testing In Vitro Breakthroughs And Trends In Cell Culture Technology Wiley

Ivd Testing 5 Reasons In Vitro Diagnostics Are Superior

In Vitro Toxicology Testing Market End Use And Pestel Analysis By 22 Report Survey

In Vitro Drug Testing Diag2tec Preclinical Cro

What Are In Vitro Diagnostic Tests And How Are They Regulated The Pew Charitable Trusts

Alternatives Stop Animal Testing

Genetic Toxicology Eurofins Medical Device Testing

In Vivo Vs In Vitro Drug Development Youtube

Feed Quilting Testing Measuring In Vivo In Vitro Digestibility Global Aquaculture Advocate

In Vitro Toxicity Testing

In Vitro Release Testing Ivrt Absorption Systems
In Vitro Technology Developers Step Up To Help With Coronavirus Research And Testing

Patch Testing Unavailable How In Vitro Can Help In Testing Times Xcellr8

In Vitro Testing Medical Device Biocompatibility Evaluations Full Biocompatibility Testing Program Aps American Preclinical Services Minneapolis Mn

Global In Vitro Toxicology Testing Market Ken Research

Choosing The Right In Vitro Test For Your Monoclonal

In Vitro Alternative Testing Methodology Fundacio Bosch I Gimpera

Liver Spheroids A Major Step Towards Meaningful In Vitro Testing For Human Hepatotoxicity Potential Technology Networks

Advanced In Vitro Methodologies Demonstrate New Paradigms For Toxicological Testing Technology Networks

In Vitro Testing Wickham Laboratories Uk Testing Laboratory

Pharmaceutical Stability Testing And In Vitro Diagnostics

New Animal Free Monocyte Activation Test For In Vitro Pyroge

How Is In Vitro Testing Done In The Cosmetics Industry Bioalternatives

In Vitro Testing Wickham Laboratories Uk Testing Laboratory

In Vitro Vs In Vivo Preclinical Drug Testing

In Vitro Testing In Cosmetics Pros And Cons Bioalternatives
In Vitro Testing Services Qacs The Challenge Test Lab

In Vitro Diagnostic Device Support Services
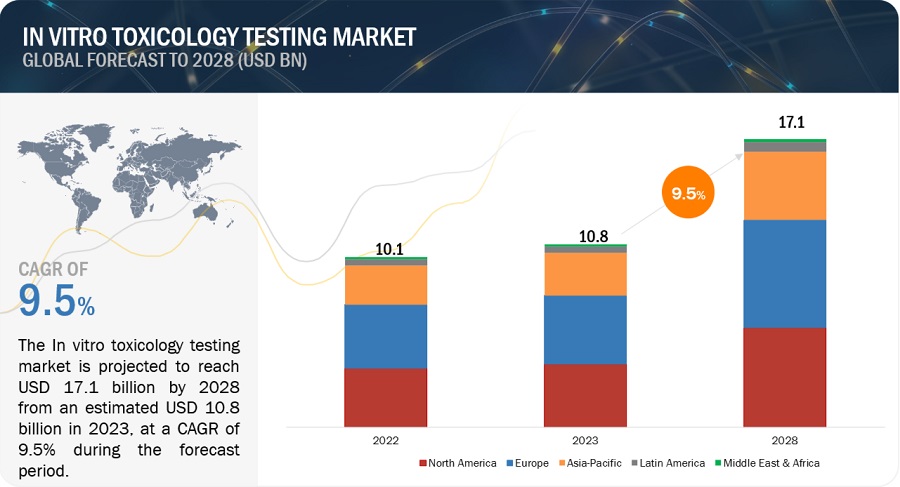
In Vitro Toxicity Testing Market Global Forecast To 25 Marketsandmarkets

Innovative In Vitro Efficacy Testing For Fungicides Sgs

Genoskin Joins 3r In Vitro Testing Platform Ivtip Genoskin
In Vitro Drug Testing Diag2tec Preclinical Cro
In Vivo Vs In Vitro What Is The Difference

In Vivo And In Vitro Testing
Research In Vitro Toxicology Testing Market By Type Product Material End User Geography 18

Reach Taking Up Alternatives To Animal Testing Kosmetica World

In Vitro Testing

In Vitro Oral Care Product Laboratory Testing
In Vitro Methods And More Animal Testing Alternatives Peta

In Vitro Testing Research For A Better Way
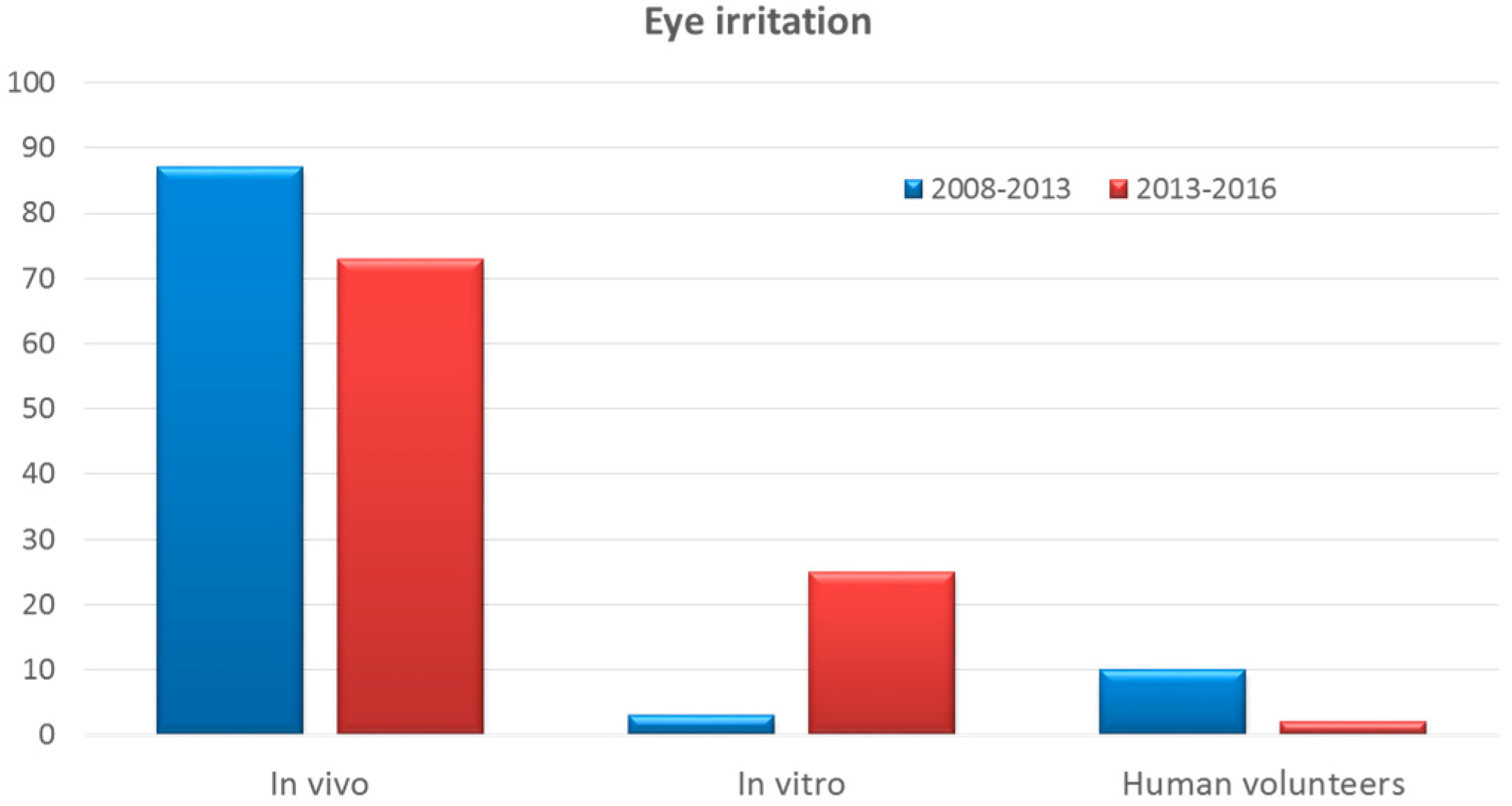
Cosmetics Free Full Text Alternative Methods To Animal Testing For The Safety Evaluation Of Cosmetic Ingredients An Overview Html
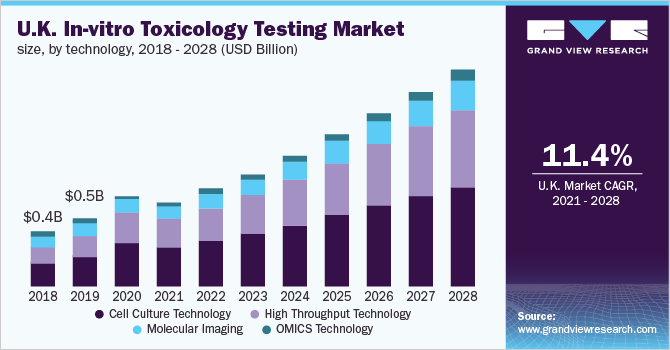
In Vitro Toxicology Testing Market Size Industry Report 27

Echa Animal Testing Alternatives Report For Reach Regulation
Cruelty Free In Vitro Breakthrough Could End Animal Testing For Carcinogenic Substances Livekindly

Echa Newsletter Home

In Vitro Wikipedia

Singapore Scientists Create In Vitro Human Skin As Alternative To Animal Testing Global Cosmetics News

In Vitro Toxicity Testing In Drug Development

In Vitro Toxicology Screening In Drug Development Admescope

In Vitro Testing Sterlab Store

A Diagram Depicting In Vivo And In Vitro Testing Stocktrek Images

Alternatives To In Vivo Biocompatibility Testing Nelson Labs

In Vitro Diagnostics Market Value To Reach 69 Billion In 24 Medical Product Outsourcing

In Vitro Release Testing Method Developed For Validation Of Semisolids

Accredited In Vitro Cytotoxicity Test Method
In Vitro Toxicity Test Creative Biolabs

Future Gazing Where Next For In Vitro Testing

In Vitro Toxicology And Toxicity Testing Market To See Cagr Of 9 3 Percent

In Vitro Toxicology Wikipedia

Vitroscreen List Of Glp Certified Tests

In Vitro Testing Abich It Biological And Chemical Analysis

In Vivo Ex Vivo And In Vitro Cosmetics Testing Protocols By Cosmetics Business Skinobs Cosmetic Testing

In Vitro Antiviral Testing Iar Usu

Uk Government Bans Animal Testing On Household Products Cyprotex

Can In Vitro Testing Replace Human Clinical Trials For Topical Drugs Contract Pharma

Fraunhofer Item In Vitro Test Methods In Inhalation Toxicology At Sot Annual Meeting Business Wire

Typical Suite Of In Vitro Techniques That Can Be Used To Evaluate The Download Scientific Diagram

Safety Methods For Eye And Skin Irritation Tests Invitrointl

In Vivo Vs In Vitro Definition Examples And More

Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd

Services For In Vitro Pyrogen Testing Sigma Aldrich

Biocompatibility Testing In Vitro Cytotoxicity Testing Surgical Materials Testing Laboratory

Incell

In Vitro Testing Merieux Nutrisciences Nederland

Animal Testing In Eu Declining Cosmetics Must Share In Vitro Methods

Iso In Vitro Diagnostic Test Systems Impact Solutions

In Vitro Drug Development And Diagnostics

How Are In Vitro Testing Methods Being Used In The Cosmetic Industry Invitrointl

In Vitro Toxicology Testing The Barriers To Progression And Adoption Technology Networks
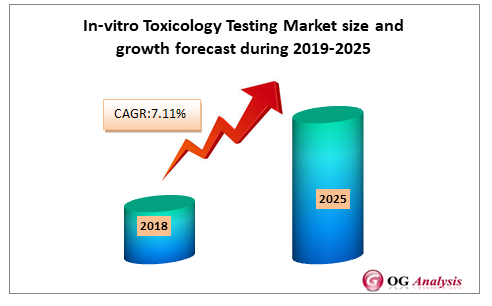
In Vitro Toxicology Testing Market 7 11 Cagr Strategic Analysis Industry Data 19 25 Oganalysis Icrowdnewswire

How In Vitro Testing Can Help Understand The Impact Of Preservatives During Prod In Cosmetics Connect



