In Vitro Dissolution Testing
Abstract The most recent ICRP respiratory tract dosimetry model (Publication 66) proposes default values for the absorption of radionuclides into blood, defin.

In vitro dissolution testing. In vitro dissolution testing is clearly a key tool for this purpose and the present bioequivalence guidelines and biopharmaceutical classification system (BCS) provides a platform for regulatory applications of in vitro dissolution as a marker for consistency in clinical outcomes. In vitro drug dissolution data generated from dissolution testing experiments can be related to in vivo pharmacokinetic data by means of in vitroin vivo correlations (IVIVC) A wellestablished. In Vitro Dissolution Absorption System (IDAS) Biopharmaceutics dissolution with better in vivo correlation The In Vitro Dissolution Absorption System (IDASTM) combines traditional dissolution testing with a means to determine and quantify interactions with a biorelevant membrane.
Use of dissolution as an in vitro test, allows it to be used as a surrogate for human/animal testing of products once a relationship has been established between the dissolution test and biological. In Vitro Tests 271 Cell Culture and Toxicity Endpoint Evaluation This could be due to the gradual dissolution of Mg 2 and Sr 2 ions in the acidic solution and the released cations react with the phosphate anions to give acid–base reactions This response of acid–base forms a network that hardens the cement faster. Segment of the lung, together with the dissolution rate of the drug product (13, 29) This review article intends to summarize recent developments in performance testing of DPIs, with a focus on selection of the relevant particle size fraction prior to dissolution testing and the application of methods for in vitro dissolution testing.
Properly designed dissolution tests will guide and accelerate drug development, assess batch to batch quality of a drug product, compare new or generic formulations with an existing product, assess the stability of the drug product, ensure the product quality in case of certain scaleup and post approval changes and provide a basis for achieving an in vitro/in vivo correlation The method should also be challenged to discriminate between batches of material with different quality attributes. The dissolution test has evolved to become a definitive tool used to characterize the performance characteristics of solid oral dosage forms As dosage forms have become more unique over the last fifty years, (in vitro) • For immediate release products. In cases where in vitro–in vivo correlations have been demonstrated, dissolution can be used as a surrogate test to predict the invivo performance of drug products (4) From a regulatory perspective, dissolution testing plays a major role in the decisionmaking process, particularly in the development and approval of generic dosage forms.
In Vitro Tests 271 Cell Culture and Toxicity Endpoint Evaluation This could be due to the gradual dissolution of Mg 2 and Sr 2 ions in the acidic solution and the released cations react with the phosphate anions to give acid–base reactions This response of acid–base forms a network that hardens the cement faster. • In vitro dissolution testing as applied to soliddosage drug forms measures the amount of drug dissolved in a known volume of liquid medium at a predetermined time, using a specified apparatus designed to carefully control the parameters of. Dissolution tests are performed with the basket method USP 1 and the Paddle method USP2 at rotational speeds of 50 and 100 rpm using a Sotax AT6 dissolution tester (Sotax, Switzerland) The dissolution medium is 500 mL of bidistilled water or simulated saliva solution, pH 67, prepared according to Peh and Choy (1999).
INTRODUCTION • Dissolution and drug release tests are invitro test s that measure the rate and extent of dissolution or release of the drug substance from a drug prod uct, usually in an aqueous medium under specifie d conditions • The dissolution test is an important quality control procedure for the drug product and is often linked to product performance in vivo. • In vitro dissolution testing as applied to soliddosage drug forms measures the amount of drug dissolved in a known volume of liquid medium at a predetermined time, using a specified apparatus designed to carefully control the parameters of. An in vitro in vivo correlation (IVIVC) is a predictive mathematical model that describes the relationship between an in vitro property of a dosage form (primarily dissolution or drug release) and a relevant in vivo response (primarily a drug’s plasma concentration or the amount of drug absorbed) 1.
In vitro dissolution testing of drugeluting stents Seidlitz A (1), Nagel S, Semmling B, Sternberg K, Kroemer HK, Weitschies W (1)Institute of Pharmacy, Biopharmaceutics and Pharmaceutical Technology, University of Greifswald, Greifswald, Germany Drugeluting stents (DES) have revolutionized the treatment of coronary artery blockage by tremendously reducing the rate of instent restenosis and the necessity of repeat revascularization compared to baremetal stents. When dissolution is the rate limiting step to absorption, the impact of formulation and process parameters on dissolution rate is key to an in vitro dissolution testing strategy The simplest method for assessing dissolution rate is a sink dissolution test. In vitro performance of appropriate test batches In the case of a generic drug product, the dissolution spccitiC.
In vitro release and dissolution testing plays a vital role in several areas during drug development Dissolution can be affected by drug substance factors (solubility, permeability, dissolution rate), dosage form factors (dissolution characteristics and manufacturing processes), and the methods used for its assessment (apparatus, method, dissolution medium). May be assessed based on in vitro dissolution tests To recommend methods for classification according to dosage form dissolution, along with the solubility and permeability characteristics of the drug substance 11 Biopharmaceutics Classification System (BCS) Solubility. During in vitro dissolution testing, the constraint of solid state interactions is removed and energy is dispersed in the larger volume of the solution Thus, with few exceptions, the entropy of solution is positive Indeed, when the heat of solution is positive, dissolution is said to be entropically driven.
The In Vitro Dissolution Absorption System (IDASTM) combines traditional dissolution testing with a means to determine and quantify interactions with a biorelevant membrane IDAS provides the ability to evaluate absorption, permeation, accumulation, biomarker regulation, and metabolism, as well as the ability to test a finished dosage form from a tablet, capsule to suspension. Annex 7 133 1033 Dissolution profile comparison for biowaivers based on dose proportionality of formulations 177 104 In vitro equivalence testing for nonoral dosage forms 177 105 In vitro equivalence testing for scaleup and postapproval changes 180 References 180 Appendix 1 Recommendations for conducting and assessing comparative. Dissolution testing is an in vitro method that characterizes how an API is extracted out of a solid dosage form It can indicate the efficiency of in vivo dissolution but does not provide any information on drug substance absorption Pharmacokinetic data supplements and provides additional information regarding API absorption rate.
1 Results from invitro dissolution rate experiments can be used to explain the observed differences in invivo availability 2 Dissolution testing provides the means to evaluate critical parameters such as adequate bioavailability and provides information necessary to formulator in development of more. In vitro dissolution testing is a critical tool to control the quality of pharmaceutical products and guide formulation development However, traditional approaches are often crude, inaccurate measures of actual intestinal release behavior in humans. Dissolution testing conducted in physiological similar conditions is an useful tool in predicting issues related to pharmacokinetics, may indicate bioequivalence between certain products as solid oral dosage forms, and is employed to optimize the development and ensure the quality of drug formulations.
Diffusion Franz vertical diffusion cells;. Therefore, quantification of in vivo plasma levels is often not feasible Due to this limitation and the fact that tissue levels cannot be determined in humans, in vitro dissolution testing is one of the most powerful tools to gain insight into the release behaviour of DES. In vitro dissolution testing plays a prominent role in assuring product performance and quality Effort should be made to investigate biorelevant dissolution testing that mechanistically resembles in vivo conditions Properly designed dissolution tests will accelerate drug development, hasten validation of postapproval changes, and possibly.
Invitro dissolution testing is a critical test that has to correlate with invivo clinical studies and which could require specific method developments Dissolution testing is described in many pharmacopeias, in EP, USP chapters and FDA guidelines USP 1, 2, 5, 6. Invitro dissolution testing can be used as waiver for invivo bioequivalence studies of generic brand interchangeability for Class I drugs ie, highly soluble and highly permeable. Dissolution test is used to forecast the invivo behaviour of a drug However, definite conclusions about the bioavailability and bioequivalence of these products should be conducted invivo studies It is critical that the invitro test should mimic the invivo conditions as closely as possible Given the nature of the human GI tract and various factors that affect its activity, the generalisation of dissolution conditions and results of this study is not recommended.
INVITRO DISSOLUTION TESTING Dissolution and drug release tests are invitro tests that measure the rate and extent of dissolution or release of the drug substance from a drug product, usually aqmedium under specified conditions It is an important QC procedure for the drug product and linked to product performance invivo NEED FOR DISSOLUTION TESTING Evaluation of bioavailability Batch to batch drug release uniformity Development of more efficacious and therapeutically optical dosage. And In Vitro Dissolution Testing US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) December 00 Clinical Medical. In vitro dissolution testing conditions that reflect and predict in vivo drug product performance are advantageous, especially for the development of paediatric medicines, as clinical testing in this population is hindered by ethical and technical considerations.
In Vitro Tests 271 Cell Culture and Toxicity Endpoint Evaluation This could be due to the gradual dissolution of Mg 2 and Sr 2 ions in the acidic solution and the released cations react with the phosphate anions to give acid–base reactions This response of acid–base forms a network that hardens the cement faster. Biorelevant disintegration and dissolution Biorelevant disintegration and dissolution tests enable you to conduct these experiments using media that simulate the appropriate gastrointestinal fluids closely Biorelevant dissolution is a generally more useful in vitro test than disintegration This is because it gives information not just on disintegration of the dosage form but also quantitative release of the drug into the surrounding simulated gut fluids. Invitro Dissolution test The drug release profile of metronidazole tablets was determined in 900ml buffer solutions of pH 12, 45 and 68 prepared according to USP at 50 rpm in apparatus II at temperature 37 °C ± 05 10ml of samples were collected after predetermined time interval ie, 5, 10, 15, 30, 45, 60, 90 and 1 min and replaced.
Stability Test of Leuprolide Acetate Solutions LA was dissolved in PBS or acetate buffer at two concentration levels, a low concentration level (001–002 mg/mL) simulating the initial concentration (time zero) of LA in the external medium of the in vitro release assay, and a high concentration level (01–02 mg/mL) mimicking the final. Invitro dissolution testing of a solid dosage form such as a fastdispersing solid dosage form, can be used for assessing batchtobatch quality of a drug product, guide development of new formulations, ensure continuing product quality and performance after changes, such as changes in the formulation, the manufacturing process, the site of. The release rate should be tested in vitro by a dissolution test method The development of a suitable dissolution test method should be based on the physicochemical in vitro and in vivo characteristics of the active substance and the drug product considering the mechanism of release.
In Vitro Dissolution Absorption System (IDAS) Biopharmaceutics dissolution with better in vivo correlation The In Vitro Dissolution Absorption System (IDASTM) combines traditional dissolution testing with a means to determine and quantify interactions with a biorelevant membrane. The pharmaceutical industry and regulatory agencies rely on dissolution similarity testing to make critical product performance decisions as part of drug product life cycle management Accordingly, the application of mathematical approaches to evaluate dissolution profile similarity is described in regulatory guidance However, the requirements (eg, which time points, number of time points. Describes current regulatory conditions for in vitro drug release testing Features contributions from well respected global experts in dissolution testing In Vitro Drug Release Testing of Special Dosage Forms will find a place on the bookshelves of anyone working with special dosage forms, dissolution testing, drug formulation and delivery, pharmaceutics, and regulatory affairs.
In vitro dissolution/release tests are an indispensable tool in the drug product development, its quality control and the regulatory approval process Mucosal drug delivery systems are design ed to. Dissolution testing should be carried out under mild test conditions, basket method at 50/100 rpm or paddle method at 50/75 rpm, at 15minute intervals, to generate a dissolution profile. Watson’s Proposed InVitro Dissolution Test Conditions Dissolution Apparatus USP Type 2 Paddle Dissolution Medium 50 mL of methanol to 950 mL of water Stirring Speed 75 rpm Temperature.
In Vivo Predictive Dissolution Testing of Montelukast Sodium Formulations Administered with Drinks and Soft Foods to Infants Abstract In vitro dissolution testing conditions that reflect and predict in vivo drug product performance are INTRODUCTION Understanding the dissolution profile of a. Chromatography HPLC, UPLC, Ion Chromatography (IC), and Size Exclusion (SEC) Detection by UV, MS, RID, MALS, CAD, and Fluorescence;. Chapter , Assessment of drug product performance—bioavailability, bioequivalence, and dissolution, discusses the relationship of in vitro dissolution to in vivo performance It gives specific guidance on in vitro dissolution comparisons, statistical methods, and the justification of biowaivers.
Content originally posted April 16 and updated on October 23, 19 An in vitro in vivo correlation (IVIVC) is a predictive mathematical model that describes the relationship between an in vitro property of a dosage form (primarily dissolution or drug release) and a relevant in vivo response (primarily a drug’s plasma concentration or the amount of drug absorbed) 1. In vitro performance of appropriate test batches In the case of a generic drug product, the dissolution spccitiC. Dissolution Testing Guide product design Quality control testing Product to product performance comparison Develop invivo / invitro correlation (IVIVC) In vitro laboratory test method designed to demonstrate how efficiently an active ingredient is extracted out of a solid oral dosage into solution Applications in Pharmaceutical Industry.
Use of dissolution as an in vitro test, allows it to be used as a surrogate for human/animal testing of products once a relationship has been established between the dissolution test and biological. Dissolution tests with a permeation step include biphasic dissolution tests where the simulated GI media is in contact with a waterinsoluble organic phase, 5 membrane flux tests incorporating a lipidfilled membrane (membrane is impermeable to micelles, inclusion complexes, etc), 6 and dissolution/permeation systems that incorporate Caco2 or other cell monolayers to separate the dissolution and permeation compartments 7 By using a dissolution test with a permeation step, API is constantly. Invitro dissolution testing should provide a robust body of data in order to assure product performance and quality Throughout the process it is important to ensure that the invitro dissolution resembles invivo conditions If the dissolution procedure is designed effectively, it will accelerate drug development, and ideally reduce the need.
Two main uses of invitro dissolution testing are now discussed further • to assess the quality of solid drug products (ie using invitro dissolution testing as a quality control tool) • as a prognostic tool for the performance of solid drug products in the gastrointestinal tract (known as predictive dissolution) As a quality control tool. AMRI has a wide variety of dissolution and related instrumentation to support in vitro bioequivalence testing, including USP Dissolution Apparatus 1;. In Vitro Release Testing Explained During all phases of the drug development process and lifecycle, dissolution testing, which detects physical changes in API and the drug formulation, is required for all solid oral dosage forms Similarly, for nonoral dosage forms, including semisolid topical formulations, in vitro release testing is needed to evaluate drug release properties.
In pharmaceutical Dissolution test are used for in vitro testing of the tablets and capsulesDissolution apparatus are used through the product development life cycle from product release to stability testing in the Quality Control department then after passes or approval from quality department drugs are sent to marketsdetails discussion about dissolution test and apparatus are given in. In Vitro Tests 271 Cell Culture and Toxicity Endpoint Evaluation This could be due to the gradual dissolution of Mg 2 and Sr 2 ions in the acidic solution and the released cations react with the phosphate anions to give acid–base reactions This response of acid–base forms a network that hardens the cement faster. Early Dissolution Testing in a FDA Lab in 1970s USP rotatingbasket apparatus and a centering tool USP paddle apparatus Pharmaceutical Technology, April, 1978, Vol 2, 1653 8 FDA’s Definition and Views on Dissolution Testing in 1970’s • In vitro dissolution testing as applied to soliddosage drug forms measures the amount of drug.
The aim is to correlate as closely as possible measured invitro parameters with oral bioavailability This type of dissolution testing is known as predictive dissolution testing Unlike QC dissolution testing, it may require dissolution test methods which reflect more closely the physiological makeup of the gastrointestinal tract. A recent United States Food and Drug Administration (FDA) guidance 6 describes how sponsors can apply for waivers in lieu of in vivo bioavailability and bioequivalence testing based on a combination of the biopharmaceutics classification system (BCS) and in vitro dissolution testing BCS classifies compounds into four categories based on their solubility and permeability characteristics.

Dissolution Testing Pharmaceutical Technology

Use And Limitations Of In Vitro Dissolution Testing Topic Introduction And Overview Pharmaceutical Formulation In Vitro

Top Pdf In Vitro Dissolution Profiles 1library
In Vitro Dissolution Testing のギャラリー
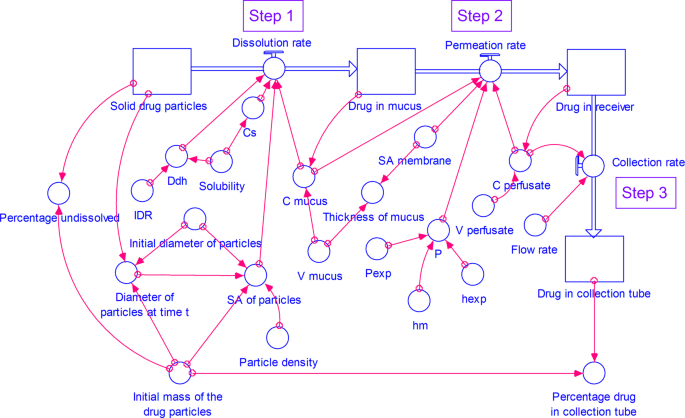
A Stella Simulation Model For In Vitro Dissolution Testing Of Respirable Size Particles Scientific Reports

Pdf Primer On The Science Of In Vitro Dissolution Testing Of Oral Dosage Forms And Factors Influencing Its Biological Relevance Semantic Scholar
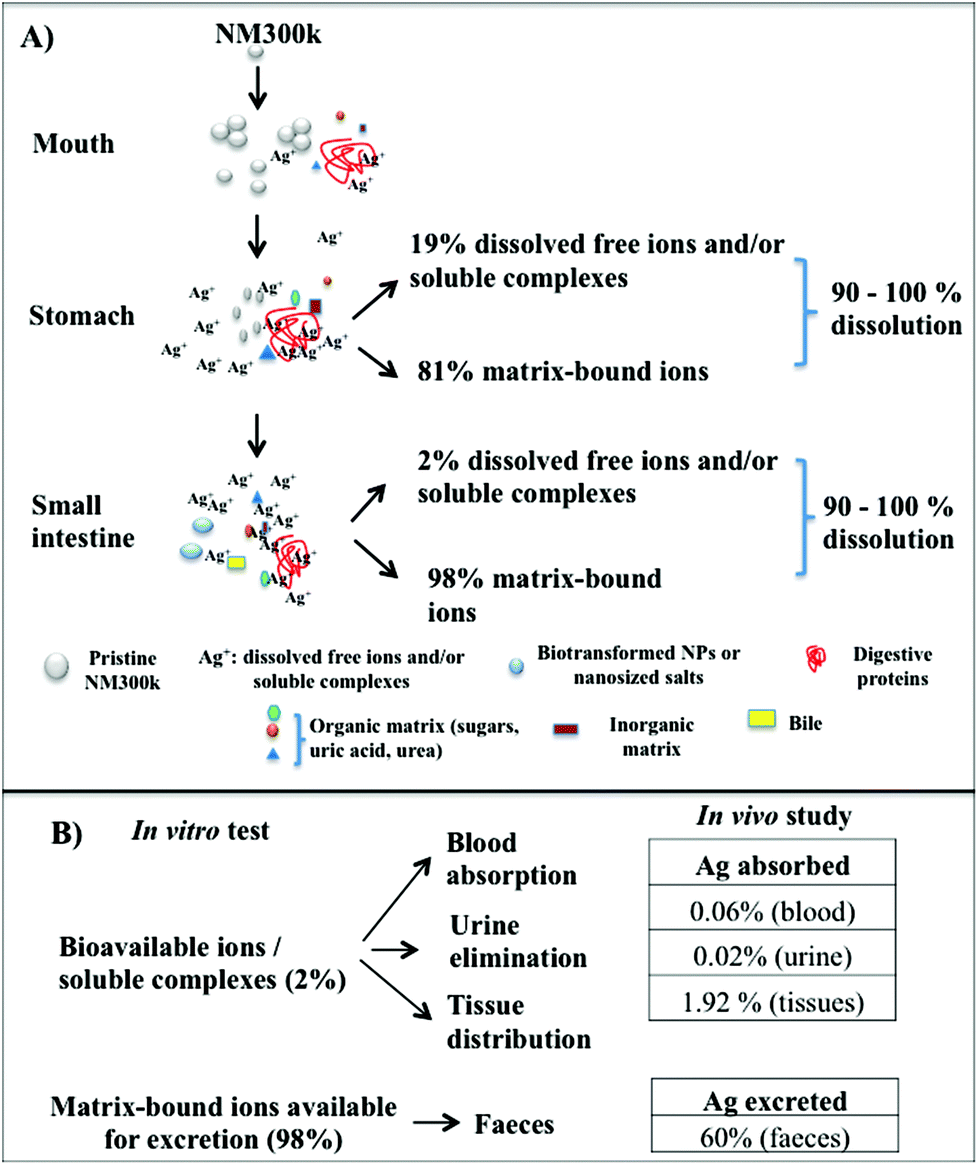
Dissolution Test For Risk Assessment Of Nanoparticles A Pilot Study Nanoscale Rsc Publishing
Dissolution Testing And In Vitro In Vivo Correlation Of Conventional And Sr Preparations Formulation Development And Optimization Is An Ongoing Process Ppt Download

Dissolution And Drug Release Testing Ppt Video Online Download
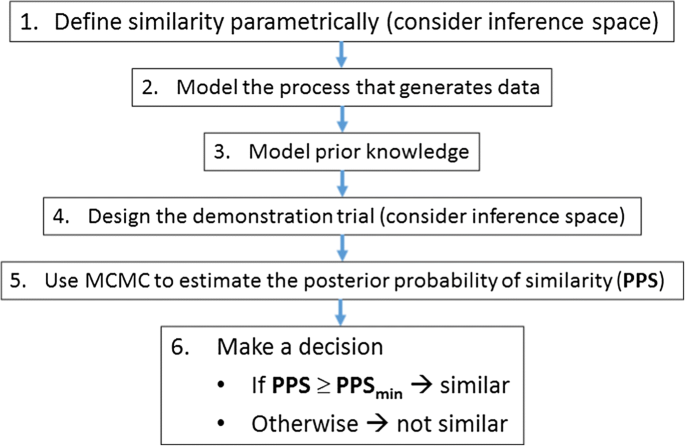
In Vitro Dissolution Profiles Similarity Assessment In Support Of Drug Product Quality What How When Workshop Summary Report Springerlink

In Vitro Dissolution Testing Models

Development And Validation Of A Dissolution Test For 6 Mg Deflazacort Tablets Topic Of Research Paper In Nano Technology Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science Hub

Investigation Of A Suitable In Vitro Dissolution Test For Itraconazole Based Solid Dispersions Sciencedirect

Pdf In Vitro Dissolution Testing Strategies For Nanoparticulate Drug Delivery Systems Recent Developments And Challenges

Biorelevant Ph Gradient M

In Vitro Dissolution Testing For Solid Oral Dosage Forms Lls Health Cdmo

Dissolution Testing For Osd Detect Physical Changes In Api And The Formulated Product Lls Health Cdmo
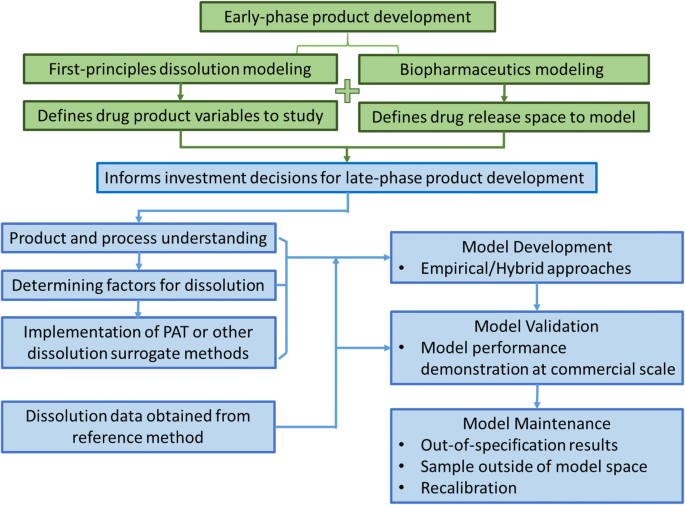
First Principles And Empirical Approaches To Predicting In Vitro Dissolution For Pharmaceutical Formulation And Process Development And For Product Release Testing Springerlink
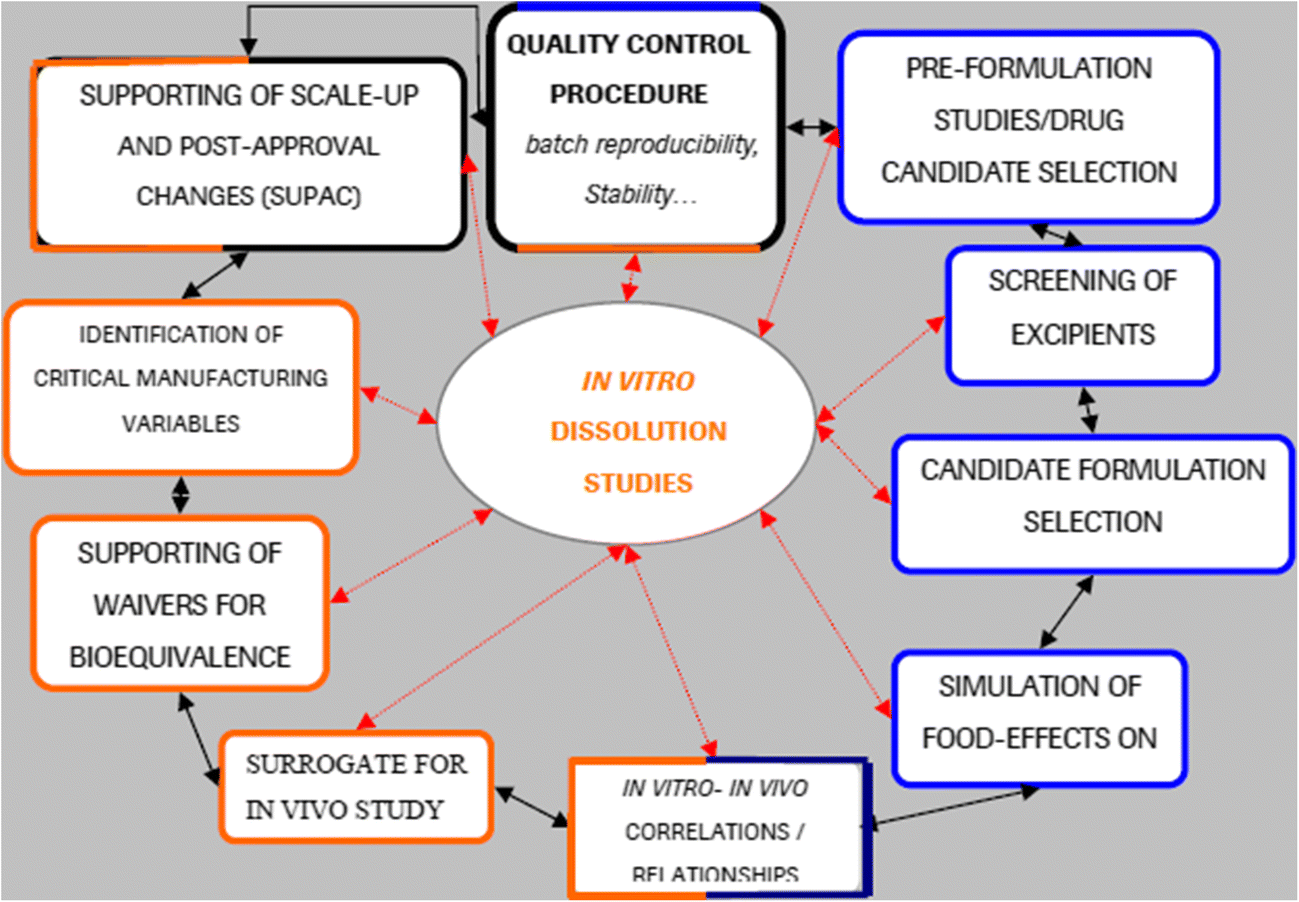
Figure 1 In Vitro Drug Dissolution Permeation Testing Of Nanocarriers For Skin Application A Comprehensive Review Springerlink

In Vitro Dissolution Testing Models

In Vitro Dissolution Testing Of Oral Thin Films A Comparison Between Usp 1 Usp 2 Apparatuses And A New Millifluidic Flow Through Device Sciencedirect

Roles Of In Vitro Dissolution Testing In Pharmaceutical Drug Development Download Scientific Diagram

Reimagine The In Vitro Dissolution Experiment With Dddplus 5 0 Youtube

In Vitro Dissolution Testing For Therapeutic Equivalence Of Metronidazole Immediate Release Tablets Available In Karachi Pakistan

Seminar On In Vitro Dissolution Testing Models Ppt Download

Figure 1 From In Vitro Dissolution Testing Strategies For Nanoparticulate Drug Delivery Systems Recent Developments And Challenges Semantic Scholar

In Vitro Dissolution Tests Gelatin Capsules Farmacapsulas

Pdf Flow Through Dissolution Testing A Comparison With Stirred Beaker Methods Claude Farrugia Academia Edu

In Vitro Dissolution Testing Models

Dissolution Specifications For Oral Drug Products Ir Dr Er In The Usa A Regulatory Perspective Issuu
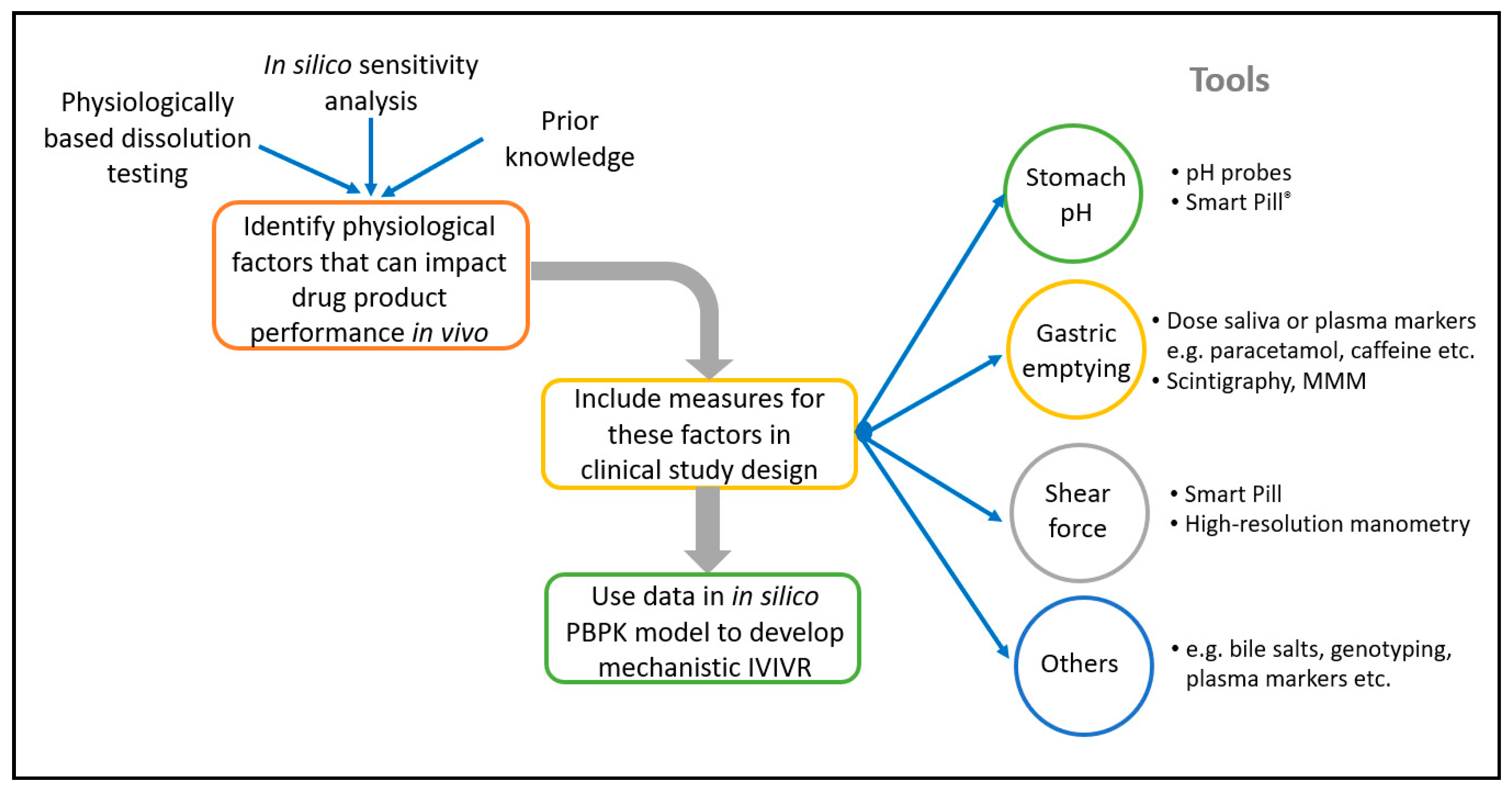
Pharmaceutics Free Full Text Developing Clinically Relevant Dissolution Specifications For Oral Drug Products Industrial And Regulatory Perspectives Html

Full Text A Novel Dissolution Media For Testing Drug Release From A Nanostructur Ijn

Study Of The Pepsin Enzymatic Activity In In Vitro Dissolution Test Of Bromazepam Tablets By Uv Vis Spectrophotometry Docsity

In Vitro Dissolution Testing For Solid Oral Dosage Particle Sciences

Disintegration In Vitro Dissolution And Drug Release Kinetics Profiles Of K Carrageenan Based Nutraceutical Hard Shell Capsules Containing Salicylamide In Open Chemistry Volume 18 Issue 1

Investigating The Impact Of Drug Crystallinity In Amorphous Tacrolimus Capsules On Pharmacokinetics And Bioequivalence Using Discriminatory In Vitro Dissolution Testing And Physiologically Based Pharmacokinetic Modeling And Simulation Journal Of

Development And Optimization Of An In Vitro Dissolution Test For Orally Inhaled Drug Products

Progressive Applications Of Dissolution Its Impact And Implications In The Pharmaceutical World Shah 13 Journal Of Pharmaceutical Sciences Wiley Online Library

Archived Issues

Dissolution Testing Wikipedia

Dissolution Dissolution Chemistry Solubility
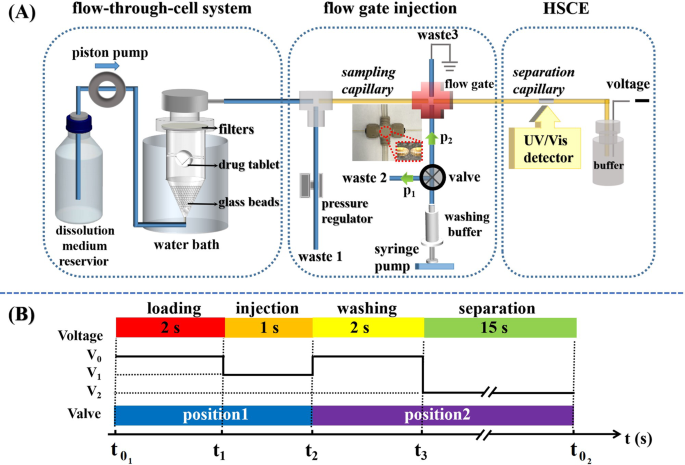
Automatic Dissolution Testing With High Temporal Resolution For Both Immediate Release And Fixed Combination Drug Tablets Scientific Reports

Ppt A Discriminating Uv Spectrophotometric Method For In Vitro Dissolution Study Of Sertraline Hydrochloride In Tablet Dosag Powerpoint Presentation Id

Dissolution Testing For Osd Detect Physical Changes In Api And The Formulated Product Lls Health Cdmo

Media For In Vitro Dissolution Testing Of Polysaccharide Based Cdds Dissolution Media With Colonic Probiotics Kotla Niranjan Goud Y Yashwant Shivapooja Ashwini Amazon Com Books

Dissolution Method Development For Fixed Dose Combination Drug Products Challenges And Strategies American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
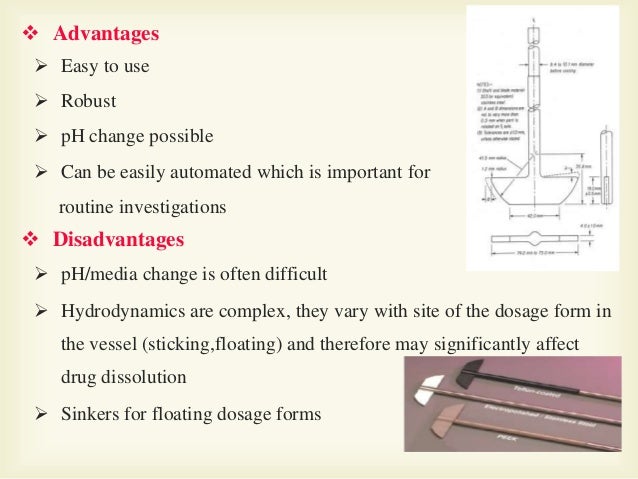
In Vitro Dissolution Testing Models

Pdf Primer On The Science Of In Vitro Dissolution Testing Of Oral Dosage Forms And Factors Influencing Its Biological Relevance Semantic Scholar
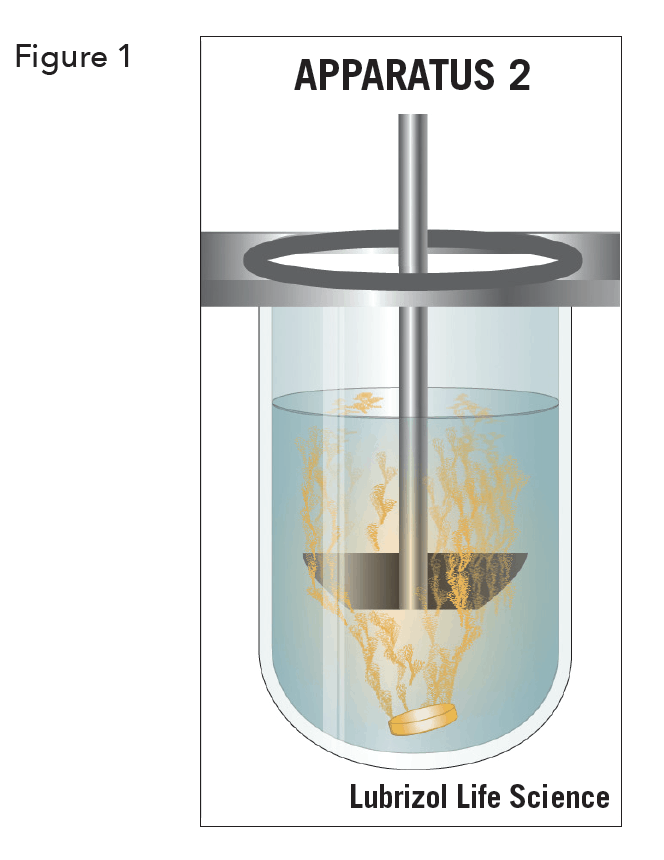
Dissolution Testing For Osd Detect Physical Changes In Api And The Formulated Product Lls Health Cdmo

In Vitro Dissolution Testing Model Authorstream
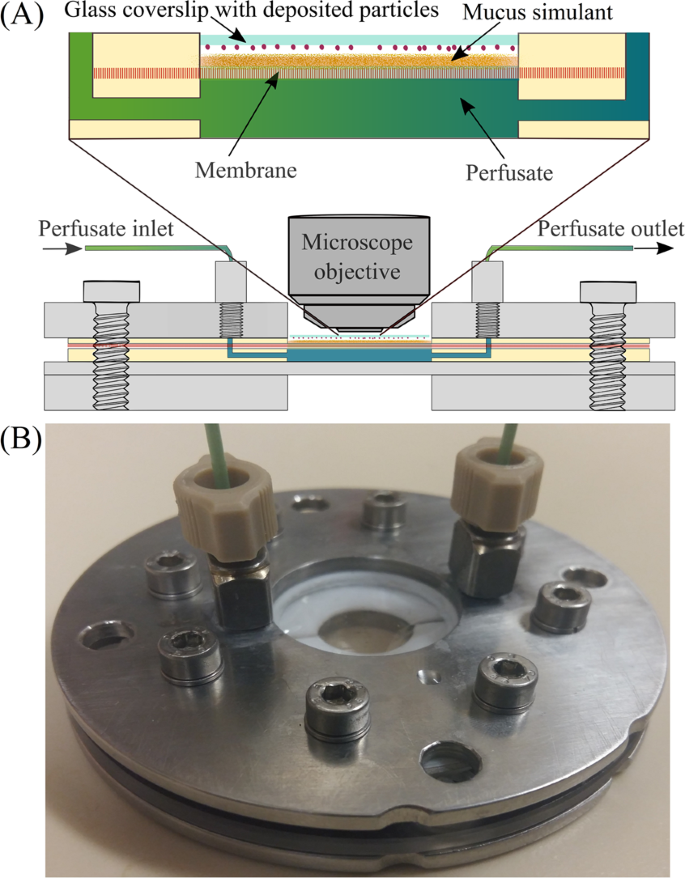
A Stella Simulation Model For In Vitro Dissolution Testing Of Respirable Size Particles Scientific Reports

Figure 2 From In Vitro Dissolution Testing Strategies For Nanoparticulate Drug Delivery Systems Recent Developments And Challenges Semantic Scholar

Fillable Online In Vitro Dissolution Profile Study Of Mucolytic Drug Ambroxol Hydrochloride From Solid Oral Dosage Form By Uhplc Ms Ms In This Paper A Simplified Dissolution Test Was Performed For The Release Of

Optimization Of An In Vitro Dissolution Test Method For Dissolution Test Method For Inhalation Formulations Pdf4pro

In Vitro Dissolution Test System For Inhaled Powder

Pharmaceutical Dissolution Testing Mark Powell Scientific Ltd

Pdf In Vitro Dissolution Testing Of Ibuprofen Using Compendial And Biorelevant Dissolution Media

Dissolution Testing How Does It Work Youtube

Selecting In Vitro Dissolution Tests For Bioavailability Enhancing Oral Formulations American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology

Development And Validation Of A Dissolution Test With Reversed Phase Liquid Chromatography Analysis For Rupatadine In Tablet Dosage Forms

Dissolution Ppt Authorstream

Comparative Dissolution Profile A Quality Control Tool And Beyond Sgs

Developing An In Vitro Dissolution And Release System For Orally Inhaled Drug Products Ondrugdelivery

In Vitro Dissolution Testing Of Respirable Size Anti Tubercular Drug Particles Using A Small Volume Dissolution Apparatus Sciencedirect

Download Ebook Media For In Vitro Dissolution Testing Of Polysaccharide Based Cdds

In Vitro Dissolution Testing Models

Outline Of Drug Release During In Vitro Dissolution Testing Of Download Scientific Diagram

Media For In Vitro Dissolution Testing Of Polysaccharide Based Cdds 978 3 8484 87 0 By Niranjan Goud Kotla Yashwant Y Ashwini Shivapooja

In Vitro Dissolution Tests Gelatin Capsules Farmacapsulas

In Vitro In Vivo Link

In Vitro Dissolution Testing Models

Development Of A Discriminative Biphasic In Vitro Dissolution Test And Correlation With In Vivo Pharmacokinetic Studies For Differently Formulated Racecadotril Granules Sciencedirect
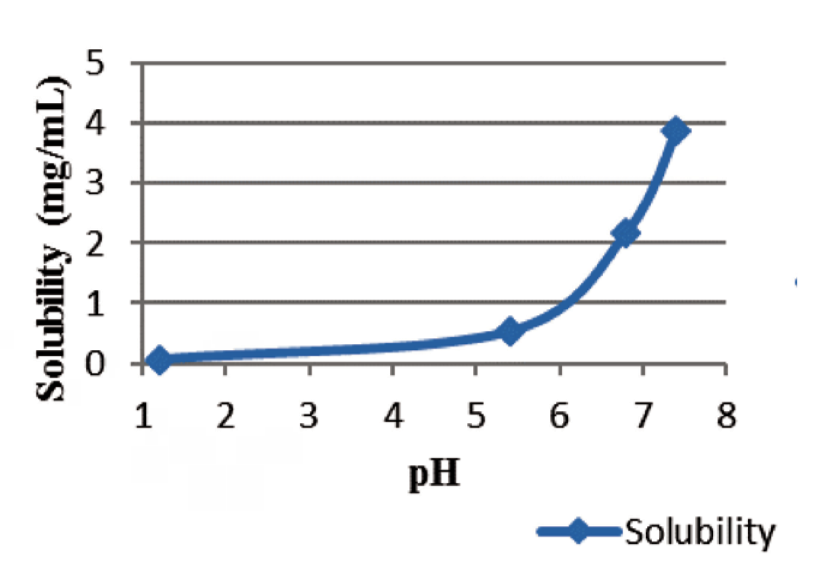
A Study On The Influence Of The Dissolution Test Factors On In Vitro Release Of Ibuprofen From Sustained Release Tablets Pharma Excipients

In Vitro Biphasic Dissolution Tests And Their Suitability For Establishing In Vitro In Vivo Correlations A Historical Review Sciencedirect

Doc Chapter 11 Dissolution Software Dibakar Das Pial Academia Edu

Modi Fi Ed Usp Xxiii In Vitro Dissolution Testing Apparatus Download Scientific Diagram
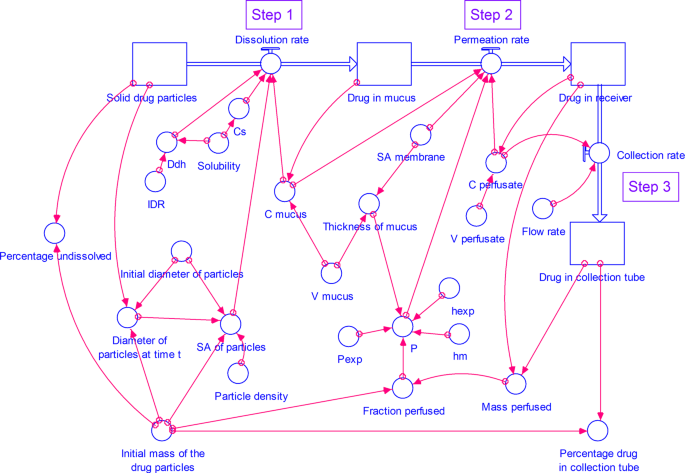
A Stella Simulation Model For In Vitro Dissolution Testing Of Respirable Size Particles Scientific Reports

In Vitro Dissolution Testing Models

The Role Of Dissolution In Drug Development

Dissolution Ppt Authorstream
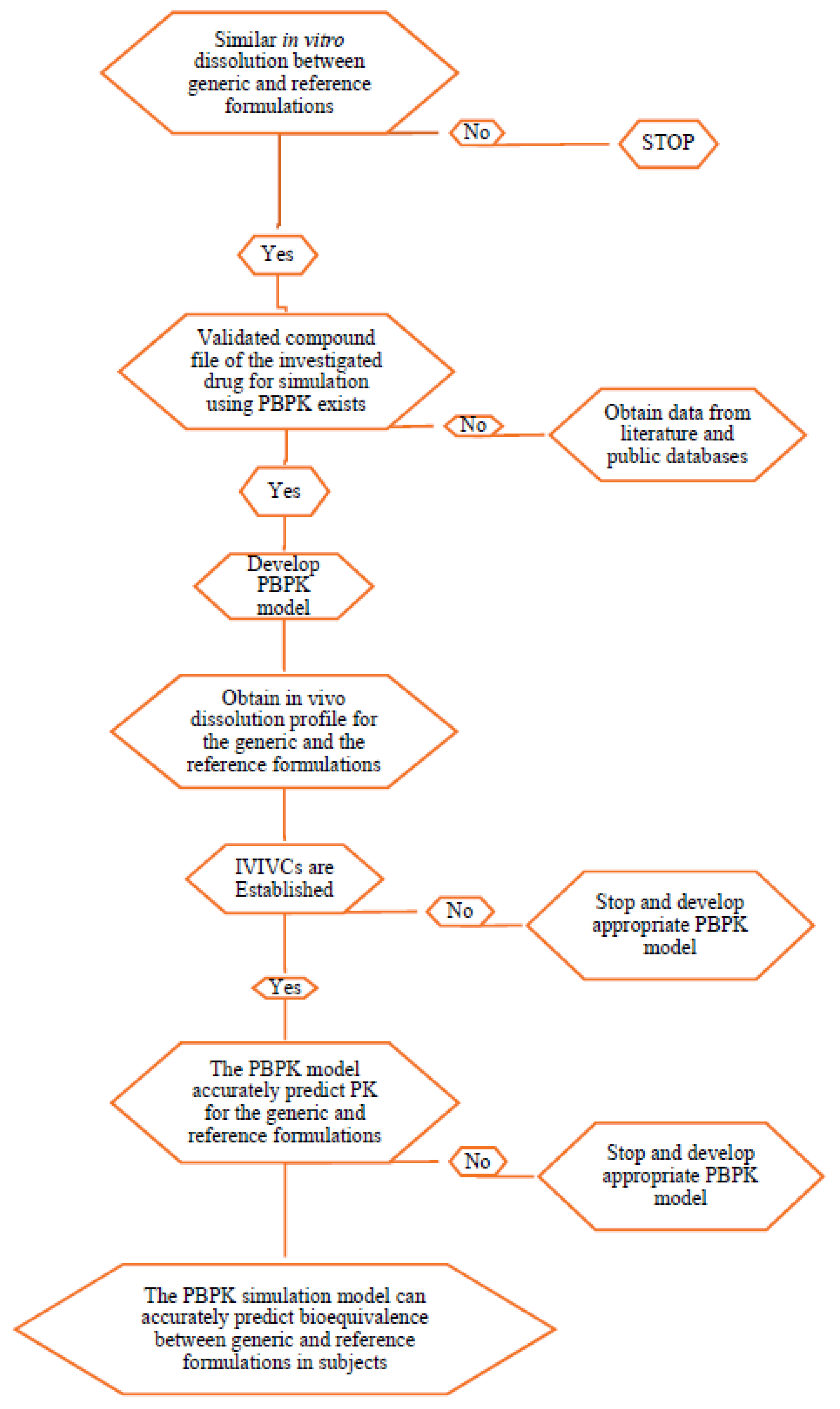
Pharmaceutics Free Full Text In Vitro Dissolution And In Silico Modeling Shortcuts In Bioequivalence Testing



