Energy Balance Equation Thermodynamics
• Or, Faculty of Mechanical Engineering, UiTM Idris Saad • The general approximation of the steady flow process can be illustrate in figure aside.

Energy balance equation thermodynamics. Energy Balance Entropy Balance Multiplying the second equation by T 0 and subtracting it from the first one yields, (1) A closed system contains internal, kinetic, and potential energies At state 1 and state 2, the total energy in a closed system is,. The first law of thermodynamics for the closed system in differential form is dU=dQdW, if the potential and kinetic energy are ignorable Here we need a sign convention to determine in whisch case heat and work assume positive values or negative values By convention, heat and work is positive when they are coming into the system. Thermodynamics, science of the relationship between heat, work, temperature, and energy Thermodynamics deals with the transfer of energy from one place to another and from one form to another The key concept is that heat is a form of energy corresponding to a definite amount of mechanical work.
H 2 O (s) H 2 O (l) D H = 600 kJ A thermochemical equation has two parts a balanced chemical equation and the change in one or more thermodynamic quantities (eg, temperature, energy, or enthalpy) that occurs when that change occurs The balanced equation can describe either a physical change (as in the example shown) or a chemical change. Thermodynamic calculations indicate the exiting enthalpy from the turbine is 1,0809 Btu/lbm (steam quality is 93 percent) Equation 2 (the first law, steadystate energy equation) becomes for the. Energy balance, constant pressure The energy balance for the constantpressure case follows from Equation 615 C P dT dt = H R kn A in which C P = V R C^P is the total constantpressure heat capacity For an ideal gas, we know from thermodynamics that the two total heat capacities are simply related, C V = C P nR (621) 16/149.
In its simplest form, the energy balance equation is meant to represent what does (or at least should) happen to the body by looking at the difference between energy intake (from food) and energy output In it’s exceedingly simplest form, the energy balance equation is this Energy in = Energy out Change in Body Stores. The second fundamental idea in thermodynamics is the total entropy balance or the "second law" of thermodynamics Entropy is a thermodynamic property that expresses the unidirectional nature of a process and, in some sense, is "nature’s clock" For example, a cup of hot coffee at room temperature cools down instead of heating up. Since the energy of a flowing fluid per unit mass is e = h ke pe = h ‘V2/2 gz The energy balance relation for steadyflow systems first appeared in 1859 in a German thermodynamics book written by Gustav Zeuner Consider, for example, an ordinary electric hotwater heater under steady operation, as shown in Fig 5–25.
The second fundamental idea in thermodynamics is the total entropy balance or the "second law" of thermodynamics Entropy is a thermodynamic property that expresses the unidirectional nature of a process and, in some sense, is "nature’s clock" For example, a cup of hot coffee at room temperature cools down instead of heating up. • The general energy balance equations can be written as;. The Energy Equation for Control Volumes Recall, the First Law of Thermodynamics where = rate of change of total energy of the system, = rate of heat added to the system, = rate of work done by the system.
Energy balance (biology), a measurement of the biological homeostasis of energy in living systems Energy balance (energy economics), verification and analysis of emergence, transformation and use of. In connection with the internal energy equation (1910) mc T Q E pdV v ∆= − mdf (1) ie, in absence of any colder system to download thermal energy to (ie with =0), and knowing that Q energy dissipated by friction is mdf ≥0 in any process, we can lower the initial temperature of a syst. The datum for the specific enthalpy values in Fig 2917 is taken as K, which is the triple point of water, which = 001 °C, and so 0 °C is taken as the datum for all thermal energy quantities in the steadyflow energy equation This means that Celsius temperatures can be used directly, as shown in Eqns 2916 to 2918, instead of absolute values, which are essential for the ideal gas.
Let's say a steam turbine produces Wt If the energy of the steam flow entering the turbine is equal to , and the energy of the steam flow leaving the turbine is E4, and there is an energy loss o. Steady flow energy equation is obtained by applying the first law of thermodynamics to a steady flow system Steady Flow Energy Equation on Mass Basis For deriving this, we have to consider m = 1 kg/sec and all other quantities will be for per kg mass such as δW/dm and δQ/dm ∴ Equation (1) becomes,. Conservation of Energy (First Law) (VW, S & B 62) Recall, dE = dQdW For the control volume, where rate of energy transfer to the system as heat rate of work done by the system For steadystate (VW, S & B 63) so (units J/s) or Neglecting potential and chemical energy (PE and CE).
Chapter 4 The First Law of Thermodynamics for Control Volumes a) The Energy Equation for Control Volumes In this course we consider three types of Control Volume Systems Steam Power Plants, Refrigeration Systems, and Aircraft Jet Engines Fortunately we will be able to separately analyse each component of the system independent of the. The energy balance equation has served as an important tool for the study of bioenergetics It is based on one of the most fundamental properties of thermodynamics and has been invaluable in understanding the interactions of energy intake, energy expenditure, and body composition Recently, however,. Chapter 4 The First Law of Thermodynamics for Control Volumes a) The Energy Equation for Control Volumes In this course we consider three types of Control Volume Systems Steam Power Plants, Refrigeration Systems, and Aircraft Jet Engines Fortunately we will be able to separately analyse each component of the system independent of the.
• Energy balance – For any system, the energy going into the system must equal the energy coming out of the system plus any accumulation of energy in the system • Only ONE energy balance equation is written for any system (or subsystem) irrespective of the number of components in the product(s) Note Once mass and energy balance. We use a heat (energy) balance on the control surface shown in Figure 198 The heat balance states that heat convected away is equal to heat radiated into the thermocouple in steady state (Conduction heat transfer along the thermocouple wires is neglected here, although it would be included for accurate measurements) The heat balance is. The energy balance principle as given in equation (7) can be invoked in thermodynamic context of a closed system with Ein, Eout and ΔEsystem as given below Ein = Qin Win Eout = Qout Wout ΔEsystem = ΔU ΔPE ΔKE.
Thermodynamic system, boundary and surrounding” in our recent post We have also discussed the “pure substances” and also “Mass balance and energy balance fora steady flow process” in the field of thermal engineering. The first Law of Thermodynamics, in rate form, applied to a control volume (CV), can be expressed as (1) where stands for massflow rate (eg, 1bm/min or kg/min) crossing the CV boundaries, h is specific enthalpy (energy/mass), surr is the rate of heat transfer from the CV to its surroundings, and st is the rate of change of energy stored in the CV This simplified form of the First Law assumes no work producing processes, no energy generation inside the CV, and negligible kinetic and. , but the energy balance of a control mass system (one that cannot exchange mass with the surroundings), and the perfect substance model for stored thermal energy, should be kept in mind ∆ E = Q W (energy balance of a control mass) (1) ∆ E = mc v ∆ T (stored thermal energy model for a perfect substance) (2).
Calculates the cooling duty required to condense and cool acetone from 100C to 25C using an energy balance Uses both sensible and latent heat to perform the. Thermodynamics is a difficult subject for anyone This wikiHow hopes to help instruct thermodynamics students in the basics of ideal gas law and heat transfer This will be going over solving an energy balance problem that can be used in. DE = dQ− dW d E = d Q − d W Here dE is an infinitesimal change in total energy when an infinitesimal amount of heat dQ is exchanged with the system and an infinitesimal amount of work dW is done by (positive in sign) or on (negative in sign) the system The time rate of change of energy within a system is expressed.
The energy balance equation has served as an important tool for the study of bioenergetics It is based on one of the most fundamental properties of thermodynamics and has been invaluable in understanding the interactions of energy intake, energy expenditure, and body composition. The internal energy of an ideal gas is therefore directly proportional to the temperature of the gas E sys = 3 / 2 RT In this equation, R is the ideal gas constant in joules per mole kelvin (J/molK) and T is the temperature in kelvin The internal energy of systems that are more complex than an ideal gas can't be measured directly. I am going over some thermodynamics right now and I am looking at the energy balance equation which is dE/dt = mdot*j(in) mdot*j(out) Qdot Wdot(external) I understand this means that the change in energy with respect to time equals the sum of the mass energy that goes into a system, minus.
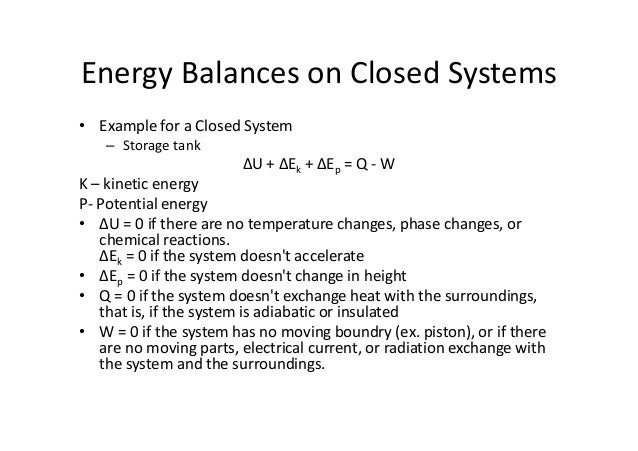
Energy Balance Entropy Balance Multiplying the second equation by T 0 and subtracting it from the first one yields, (1) A closed system contains internal, kinetic, and potential energies At state 1 and state 2, the total energy in a closed system is,. The following energy balance equation is used for a well insulating heat exchanger (Eq 8) E i n = E o u t → m ˙ 1 h 1 m ˙ 3 h 3 = m ˙ 2 h 2 m ˙ 4 h 4 This equation is meant for a heat exchanger scene in the image below If the heat exchanger you are looking at is different you may have to modify equation 8. Since additionally the system is closed, no mass flows and no energy transport across the boundary take place The general energy balance, or the 1 st Law of thermodynamics, for closed systems can be expressed as ΔU=QW or Δu=qw (per unit mass) where ΔU= the change in internal energy of this closed system;.
Note that if no mass enters or leaves the control volume during a process (mi = me = 0, and m1 = m2 = m), this equation reduces to the energy balance relation for closed systems (Fig 5–51) Also note that an unsteadyflow system may involve boundary work as well as electrical and shaft work (Fig 5–52). Another necessary equation is the law of ideal gas p= ρ∙R∙T where p=pressure;. The first law of thermodynamics, also known as the Law of Energy Conservation, tells us that energy contained in a system must equal the energy coming into it minus the energy going out of it This is a common sense idea.
• The general energy balance equations can be written as;. In it’s exceedingly simplest form, the energy balance equation is this Energy in = Energy out Change in Body Stores This is essentially just a restatement of basic thermodynamics, since energy can’t be created or destroyed, it all has to be accounted for in some form or fashion. Energy Balance for Cycles A thermodynamic cycle is a series of processes that begin and end at the same thermodynamic state The figure below demonstrates what a cycle may look like on PV coordinates (credit Zephyris CC BYSA 30, via Wikimedia Commons) At the end of a cycle, all of the properties of a substance or object (temperature, pressure, specific volume, enthalpy, etc) have the.
We notice that velocity appears in the equation of energy balance, so the conservation of mass is usually taken into consideration in order to solve the problems ρ in ∙c in ∙A in = ρ out ∙c out ∙A out where A= section area;. Energy balance, constant pressure The energy balance for the constantpressure case follows from Equation 615 C P dT dt = H R kn A in which C P = V R C^P is the total constantpressure heat capacity For an ideal gas, we know from thermodynamics that the two total heat capacities are simply related, C V = C P nR (621) 16/149. Energy balance (when temperatures are in Kelvin) m1 {c p(1) }(T 1 273) m 2 {c p(2) } (T 2 273) = m 3 {c p(3) } (T 3 273) Energy balance (when temperatures are in Celsius).
• Or, Faculty of Mechanical Engineering, UiTM Idris Saad • The general approximation of the steady flow process can be illustrate in figure aside. Also, the energy content of a control volume changes with time during an unsteadyflow process The general energy balance can be used for the control volume as E i E e = Δ E CV where E i = the total energy transferred into the control volume by heat, work, and mass E e = the total energy transferred out of the control volume by heat, work. If, as we have the entire time, we assume that the system is at steady state, we obtain the energy balance equation = This is the starting point for all of the energy balances below Consider a system in which a mass, such as water, enters a system, such as a cup, like so.
Energy Balance for Cycles A thermodynamic cycle is a series of processes that begin and end at the same thermodynamic state The figure below demonstrates what a cycle may look like on PV coordinates (credit Zephyris CC BYSA 30, via Wikimedia Commons) At the end of a cycle, all of the properties of a substance or object (temperature, pressure, specific volume, enthalpy, etc) have the. Solution to the following problem (Thermodynamics An Engineering Approach, CBK, 8th Edition, 529)Air at 600 kPa and 500 K enters an adiabatic nozzle that h. Allows us to make an energy balance that describes the energy variation of the object in Figure 21 ∆KE PE∆ = 0 21 In other words, equation 21 reads the sum of the potential and kinetic energy variation between points 1 and 2 is equal to zero Energy levels are associated with positions within a system, position 1 is the top of the.
Thermodynamic calculations indicate the exiting enthalpy from the turbine is 1,0809 Btu/lbm (steam quality is 93 percent) Equation 2 (the first law, steadystate energy equation) becomes for the. A thermodynamic system in an equilibrium state possesses a state variable known as the internal energy (E) Between two systems the change in the internal energy is equal to the difference of the heat transfer into the system and the work done by the system The first law of thermodynamics states that the energy of the universe remains the same. The change in internal, potential and kinetic energy of the system equals enthalpy goes in minus enthalpy goes out and delta Q and W 248 If we express the enthalpy and internal energy in differential form, dH equals dUPdVVdP and dU is delta QPdV Insert dU into dH equation, then dH equals delta QVdP.
DU dt dKE dt dPE dt Q_ = rate of heat transfer W_ = rate of work done;. The differential form of the energy balance can be written as a rate equation by dividing through by dt, a differential time, and then letting dt!0 in the limit to give dE dt = Q dt W dt) dE dt = Q_ W_ where dE dt = rate of energy increase within the system;.

Thermodynamics Fundamentals First Law Part 3 Energy Balance Youtube
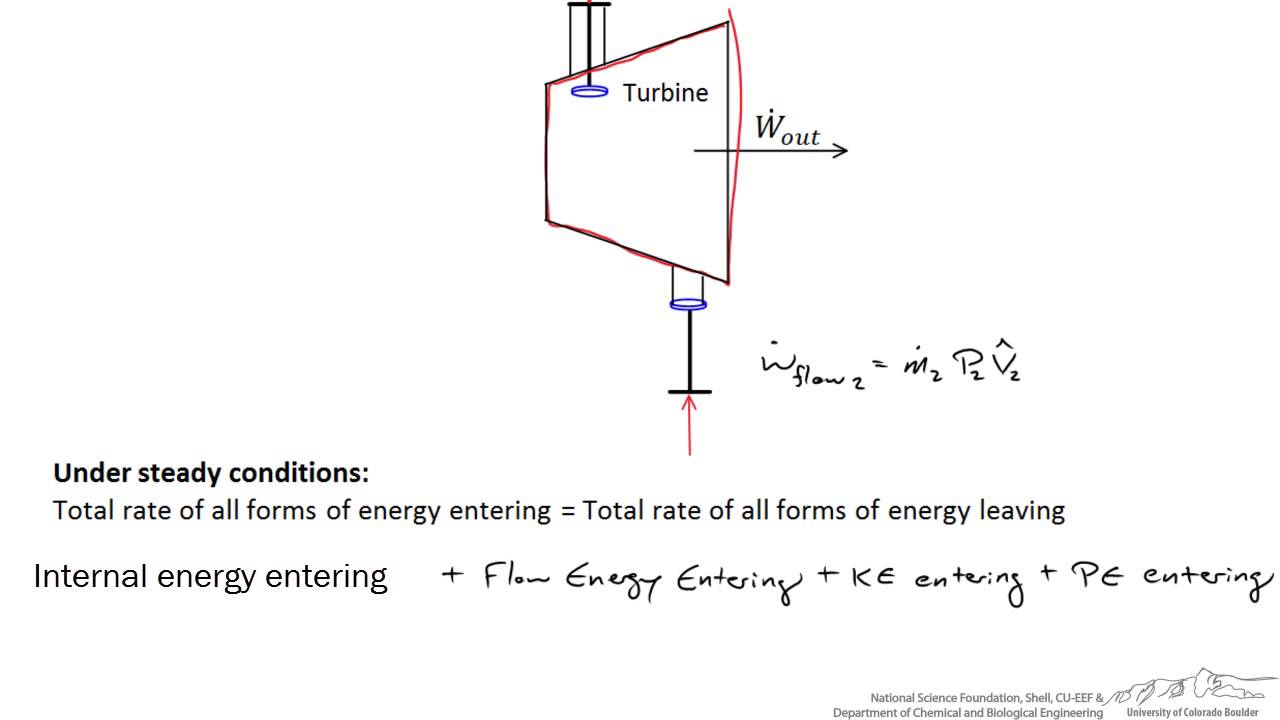
Energy Balance Around A Turbine Youtube

Examples Of The First Law Of Thermodynamics Or The Conservation Of Energy Law
Energy Balance Equation Thermodynamics のギャラリー

Exergy Balance Equation An Overview Sciencedirect Topics
Www Apogeeinstruments Com Content Lectures Principles Of Energy Balance In Environmental Systems Lecture 1 Pdf

Solved 1 Use Energy Balance Equation To Find Missing Pro Chegg Com

What Is The Sign Convention Being Used In Thermodynamics For Calculating Work Done Physics Stack Exchange

Chapter 7 Energy And Energy Balance Ppt Video Online Download

Solved Problem 6 1 Energy Balance For Steady State Open Chegg Com

86 Questions With Answers In Energy Balance Science Topic

Chapter 3a The First Law Closed Systems Energy Updated 1 17 11
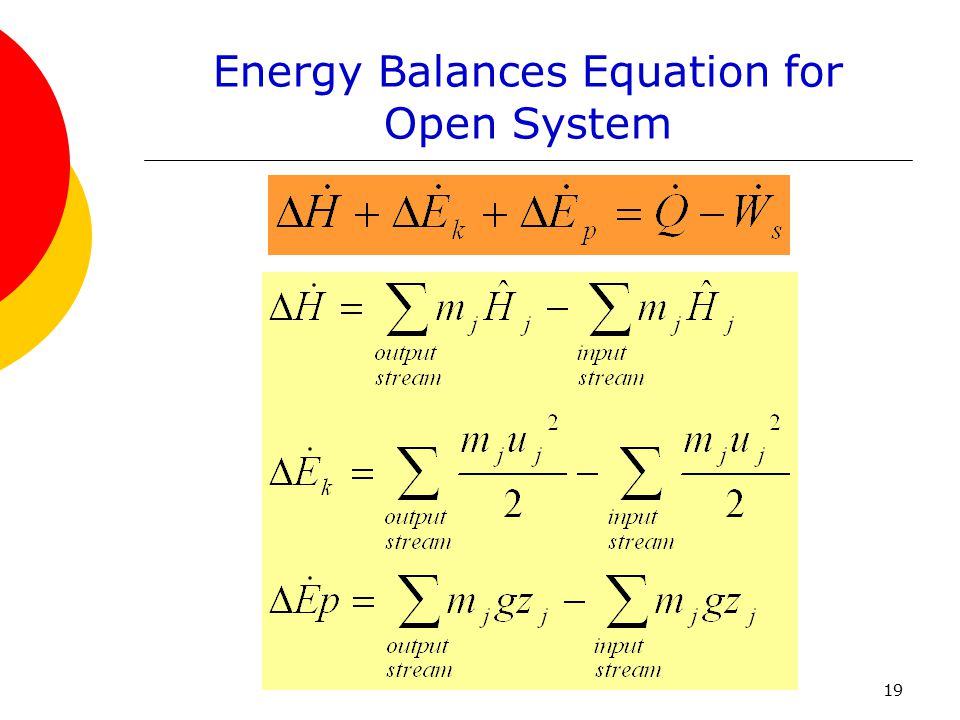
Chapter 7 Energy And Energy Balance Ppt Download
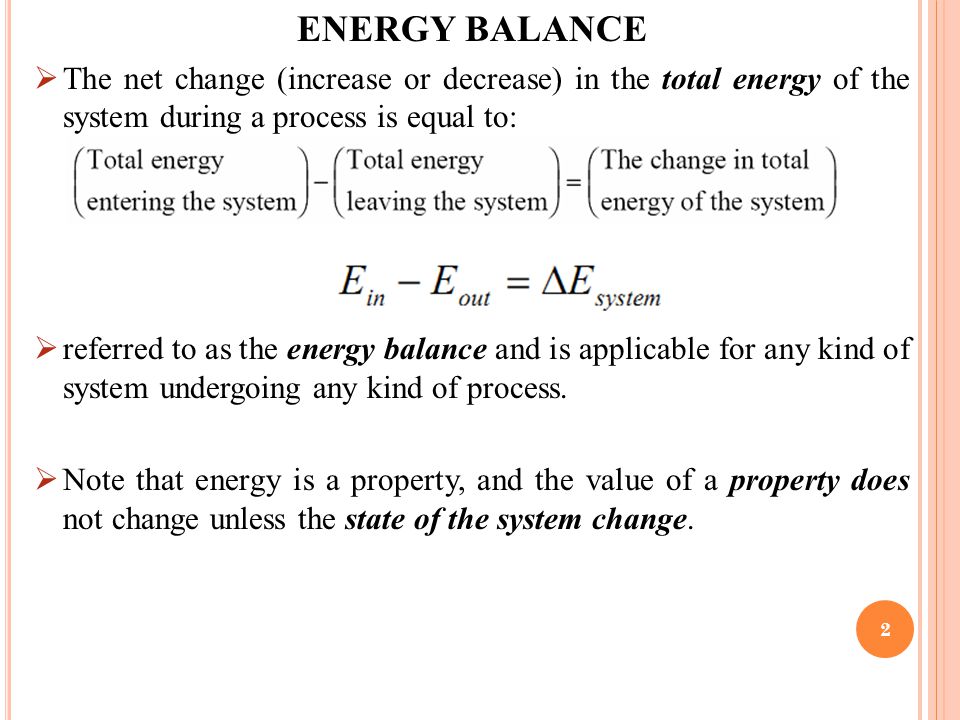
Chapter 4 First Law Of Thermodynamics Ppt Video Online Download

Thermodynamic Balance Equations Download Table
Http Faculty Poly Edu Rlevicky Handout6 Pdf
Nature Berkeley Edu Er100 Sections Week5 Section Solution Pdf
Q Tbn And9gctqymxhc 2t2ymqbks N Njgsmgumuocqxrtijfg6wbamp Xini Usqp Cau

Mass And Energy Balances
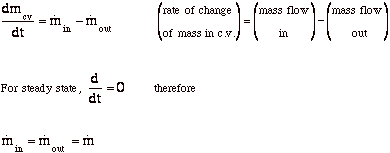
New Page 1
Q Tbn And9gcqkibshjnz9wqklwoskh3olfxi2p62pkijj67wlg21se9uohufh Usqp Cau

Energy Balance Equation An Overview Sciencedirect Topics
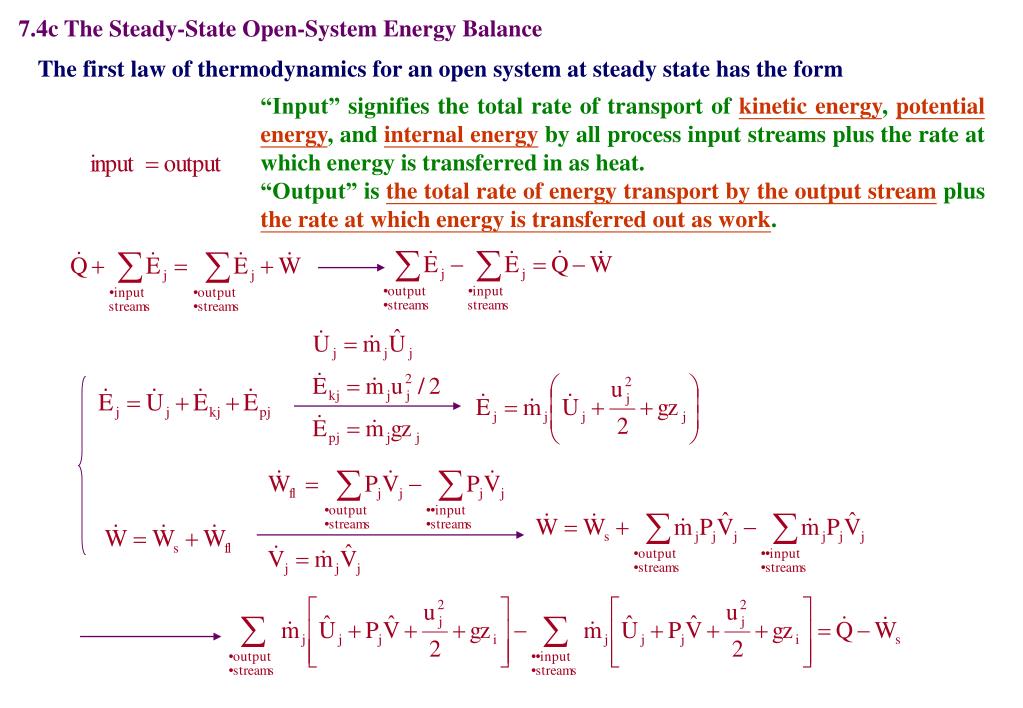
Ppt Chapter 7 Energy And Energy Balances Powerpoint Presentation Free Download Id
3

Exergy Balance Equation An Overview Sciencedirect Topics

Che Overall Energy Balance Overall Energy Balance Objectives 1 To Be Able To Derive Overall Energy Balance From The 1 St Law Of Thermodynamics 2 To Ppt Download

A Mass And Energy Balance Equations For Components Of The Fstig Cycle Download Table
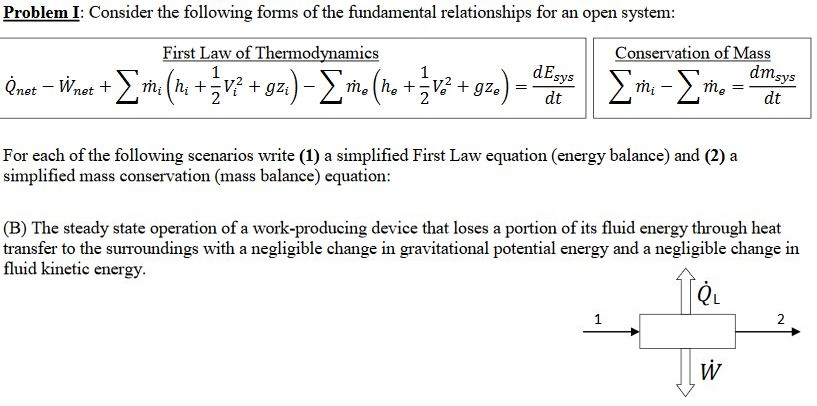
Solved Problemi Consider The Following Forms Of The Fund Chegg Com

Solved Thermodynamics Questions Write Mass And Energy Ba Chegg Com

Thermodynamics Ebook Steady Flow Devices 1
Thermodynamics Energy Balance Equation For Non Steady Flow Physics Forums
2

Jj7 Thermodynamics I Chapter 2
Thermodynamic Chapter 3 First Law Of Thermodynamics
Www Jstor Org Stable

Transient Energy Balance Derivation Youtube

Rmp Lecture Notes
Q Tbn And9gcqdffvtmmeiosatbgznaihvcnj4j7qdyaqh0ufsehmz3mbxxgzp Usqp Cau

Mass And Energy Balances

First Law Of Thermodynamics Engineers Edge Www Engineersedge Com

Why The Energy Balance Equation Results In Flawed Approaches To Obesity Prevention And Management Dr Sharma S Obesity Notes
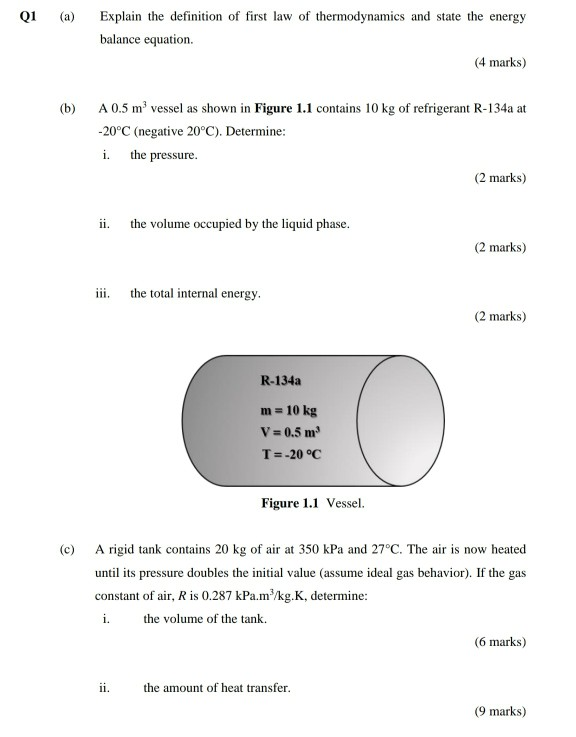
Solved Q1 Explain The Definition Of First Law Of Thermody Chegg Com
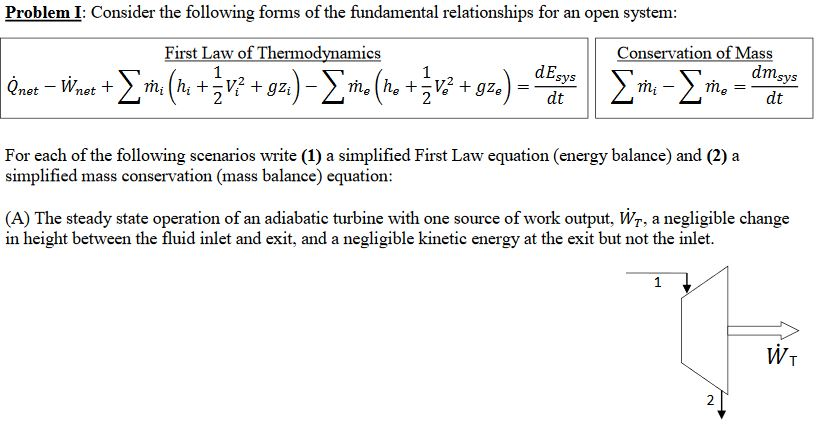
Solved Problem I Consider The Following Forms Of The Fun Chegg Com
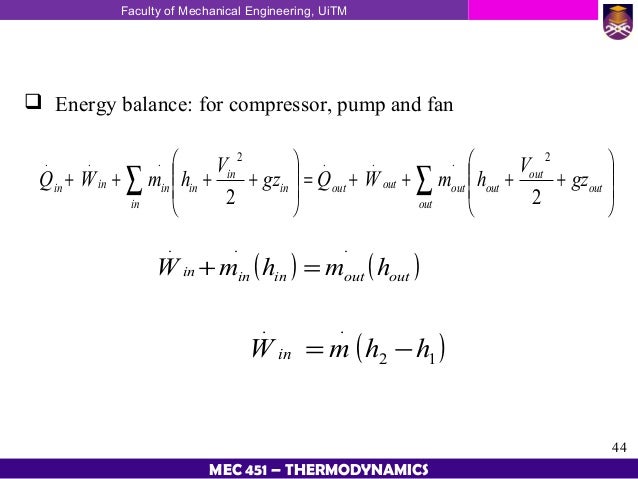
Energy Balance For The Compressor In This Figure Ppt Download

Top Pdf First Law Of Thermodynamics 1library

Thermodynamics Steady Flow Energy Balance 1st Law Turbine Youtube
02 Part5 Energy Balance

3 6 Entropy Balance Equation Test

Rmp Lecture Notes
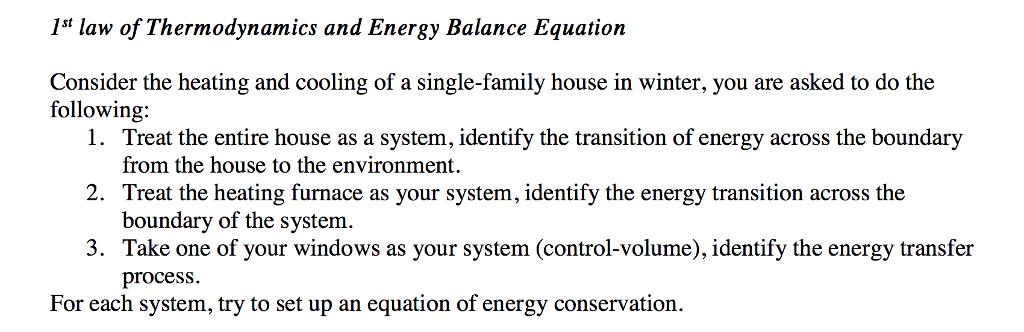
Solved Ist Law Of Thermodynamics And Energy Balance Equat Chegg Com
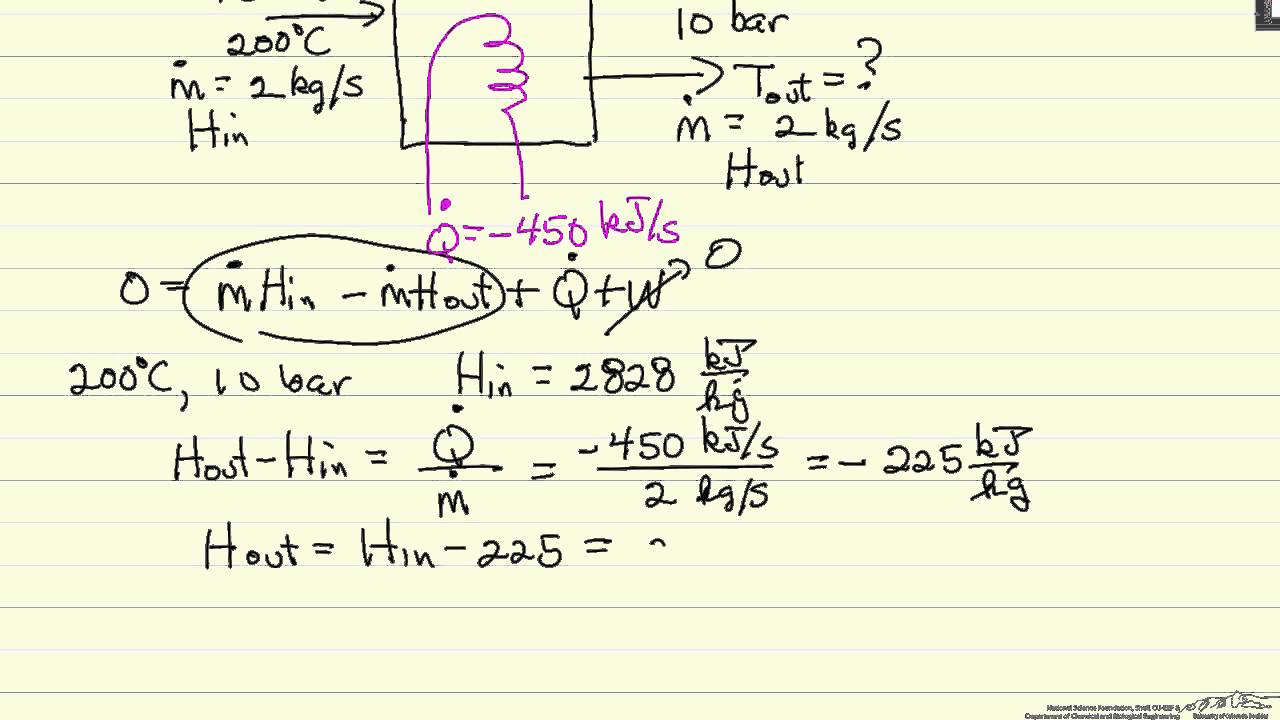
Energy Balance On A Heat Exchanger Youtube
Hello I Believe I Solved This Energy Balance Equation By Using The First Law Of Thermodynamics For An Open System Assuming Work And Heat Are Not Course Hero

Che 2 Syllabus Mass And Energy Balance Studocu

Fe Review Common Pitfalls In Thermodynamics

First Law Of Thermodynamics Engineers Edge Www Engineersedge Com
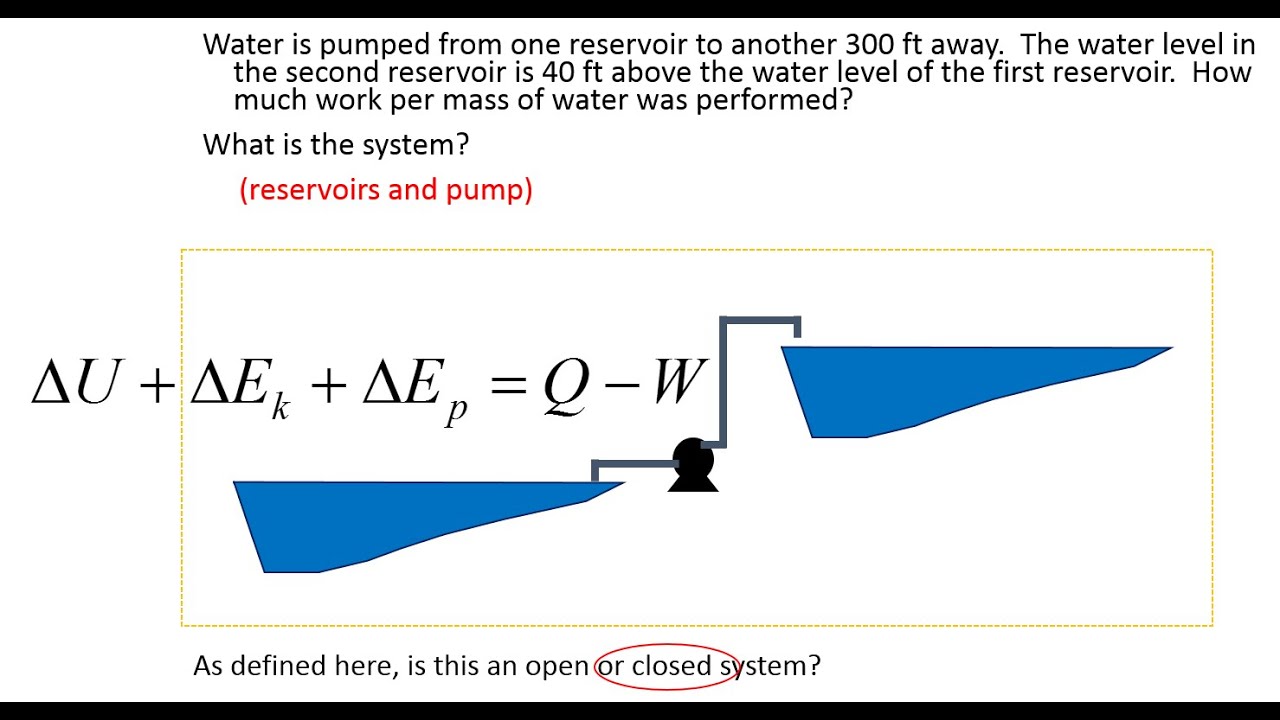
Closed System Energy Balance Youtube

Nondimensional Power Terms In Energy Balance Equation The Figure Shows Download Scientific Diagram

Chapter 2 General Mass And Energy Balance Equations Youtube
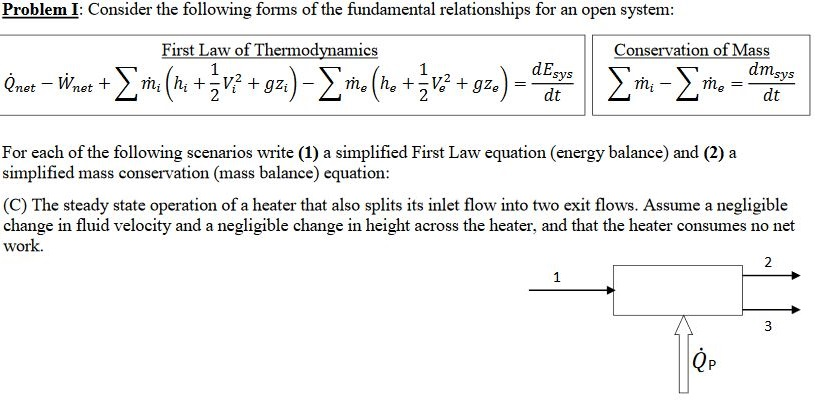
Solved Problem I Consider The Following Forms Of The Fun Chegg Com

02 Part5 Energy Balance

First Law Of Thermodynamics Engineers Edge Www Engineersedge Com

Which Software Is Better To Study About Thermodynamic Cycles In Your Opinion
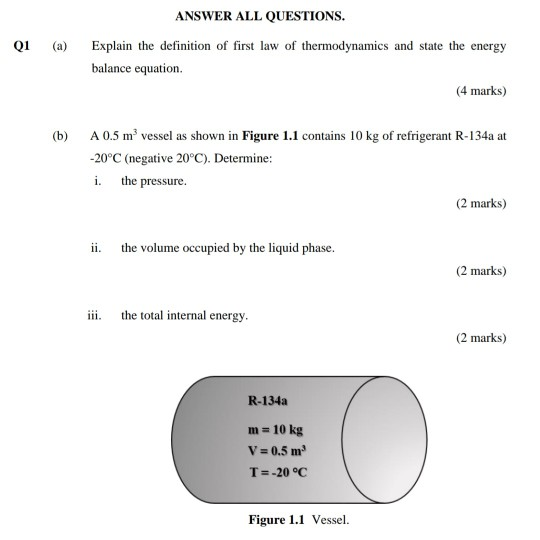
Solved Q1 A Answer All Questions Explain The Definitio Chegg Com
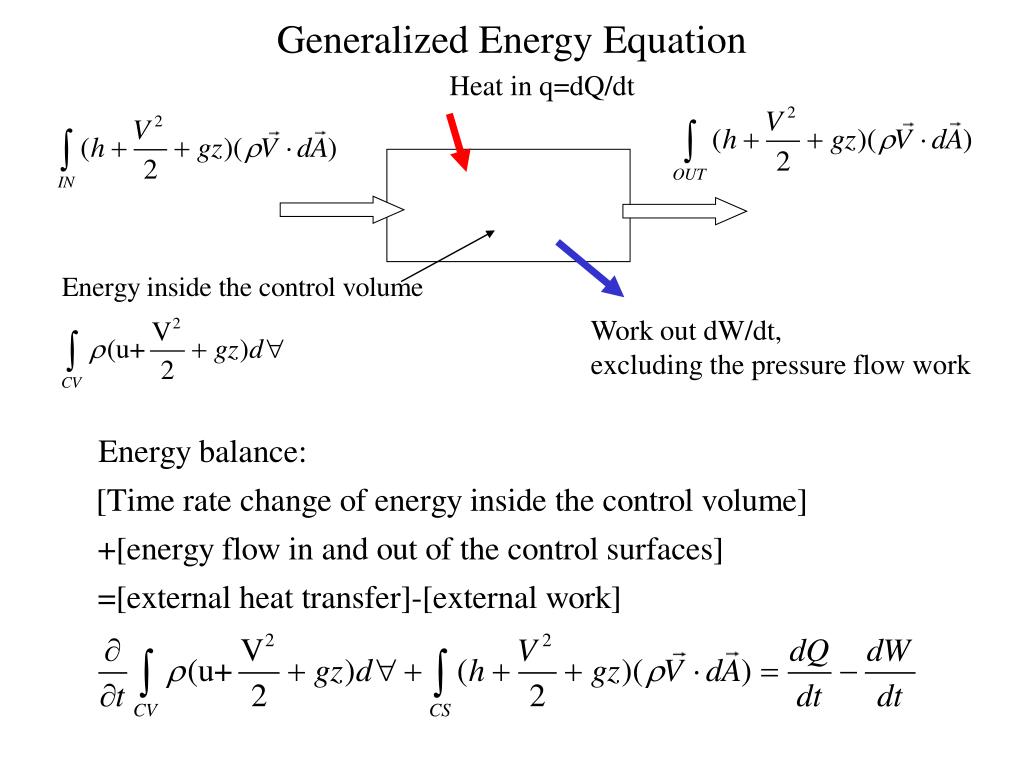
Ppt Steady Energy Equation Powerpoint Presentation Free Download Id
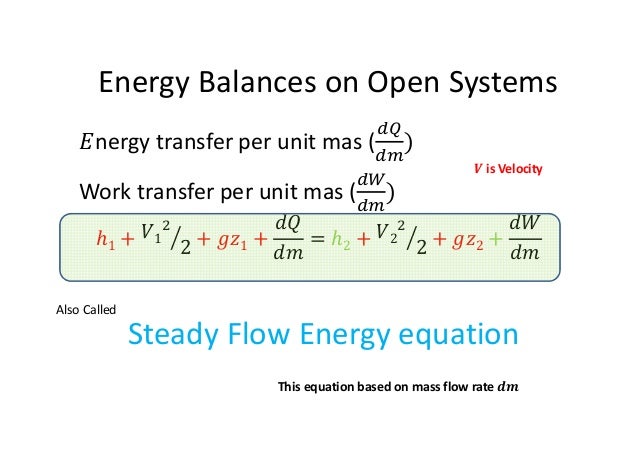
02 Part5 Energy Balance
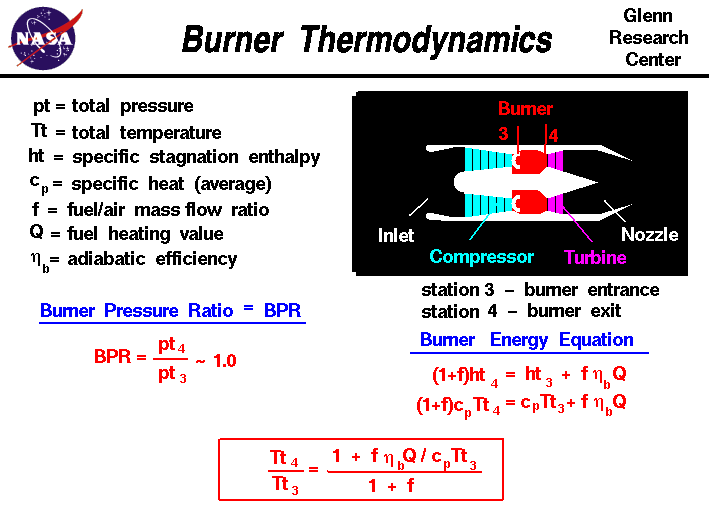
Burner Thermodynamics

Chemical Engineering Thermodynamics Laws Of Conservation Of

Solved D A The Following Time Dependent Energy Balance Chegg Com

Thermodynamics Example Mass Balance Problems Youtube

Energy Balance On A Liquid Pump Youtube
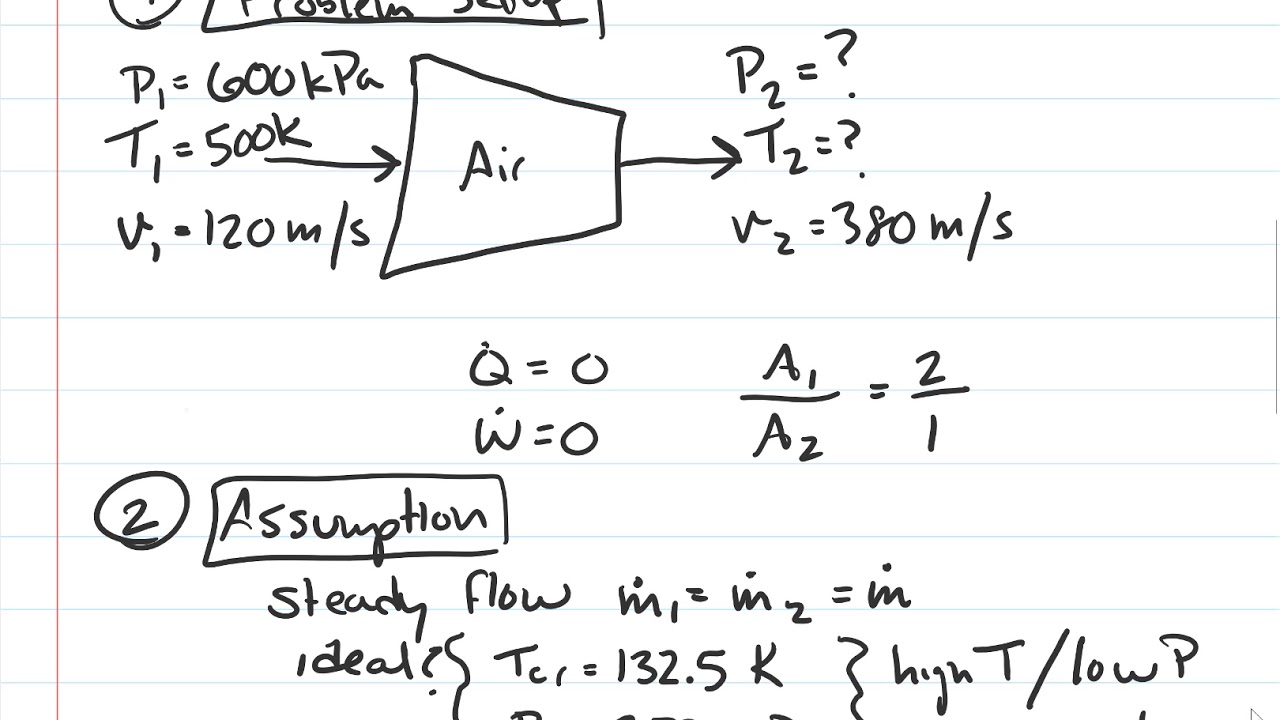
Thermodynamics Steady Flow Energy Balance 1st Law Nozzle Youtube
Thermodynamics Energy Equilibrium Ultimate Electronics Book
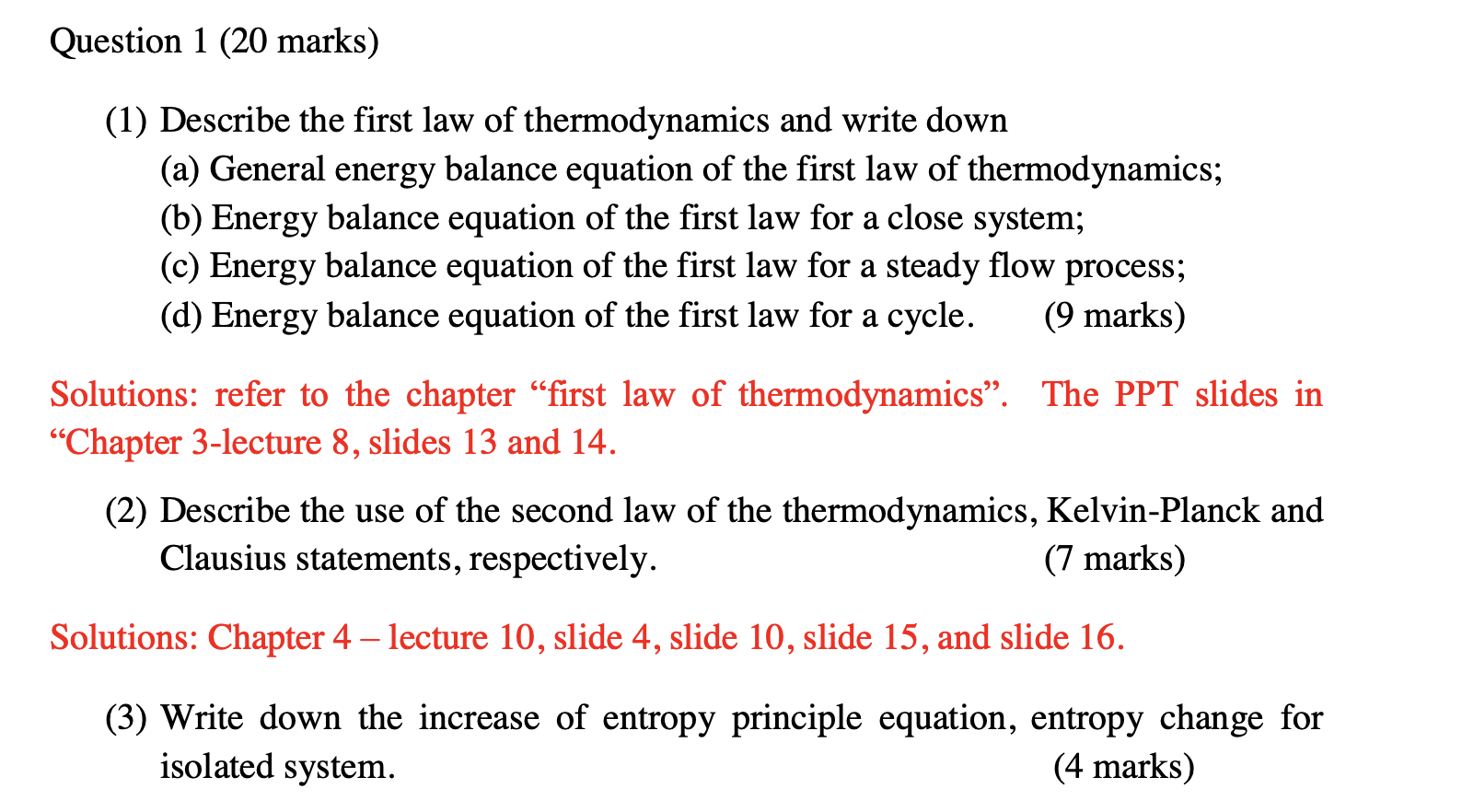
Solved Question 1 Marks 1 Describe The First Law O Chegg Com

Thermodynamics Ebook Steady Flow Process
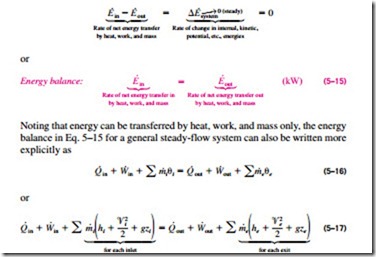
The First Law Of Thermodynamics Energy Balance For Steady Flow Systems Hydraulics And Pneumatics

Flow Process Wikipedia
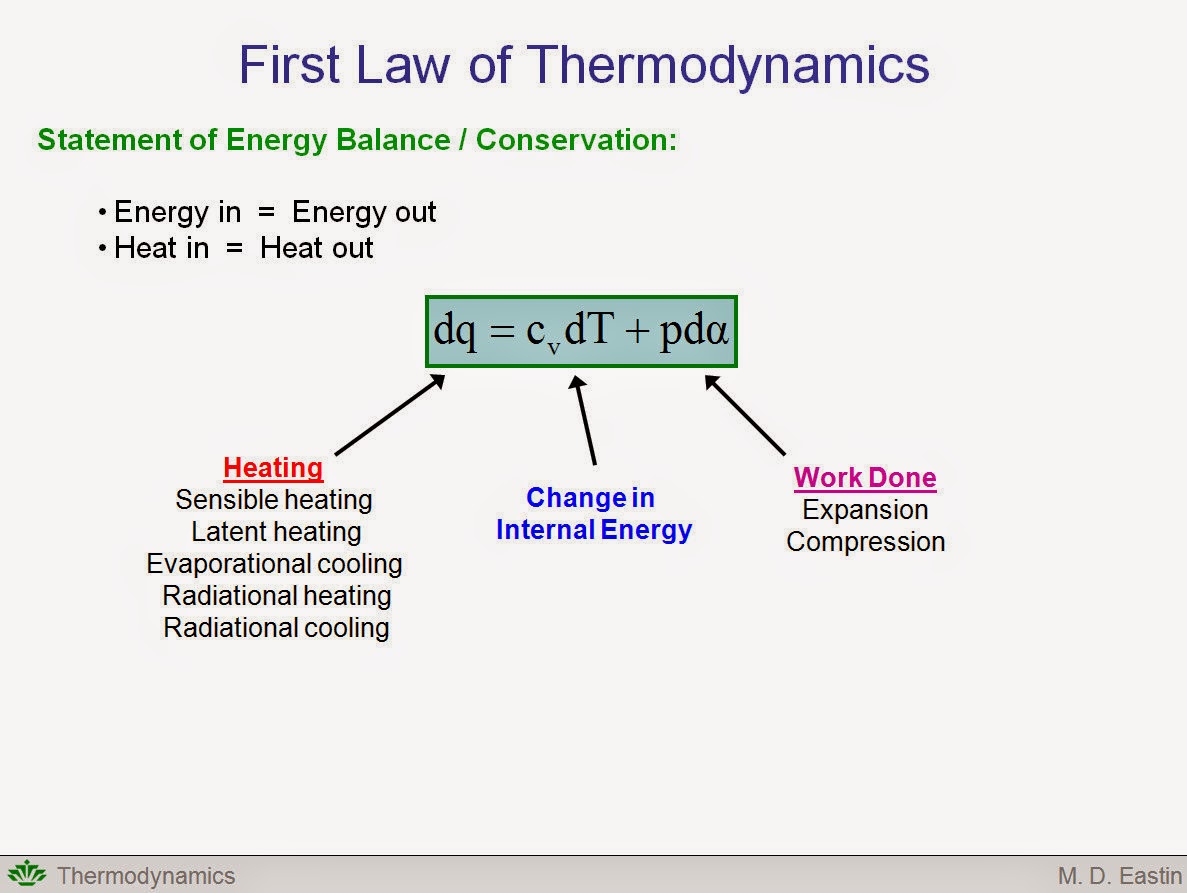
The Hockey Schtick How Gravity Continuously Does Work On The Atmosphere To Control Pressure Temperature
Energy Balance Equation For The Open System

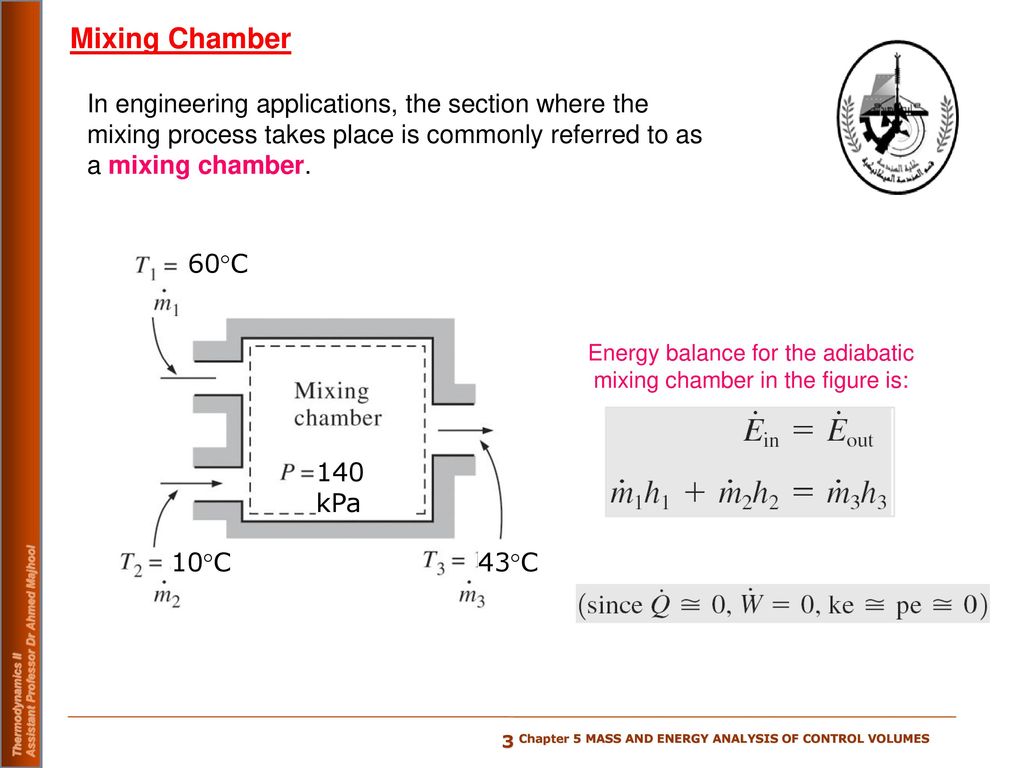
Thermodynamics Steady Flow Energy Balance 1st Law Mixing Chamber Youtube
Www Pumpfundamentals Com Download Book Chapter2 Pdf
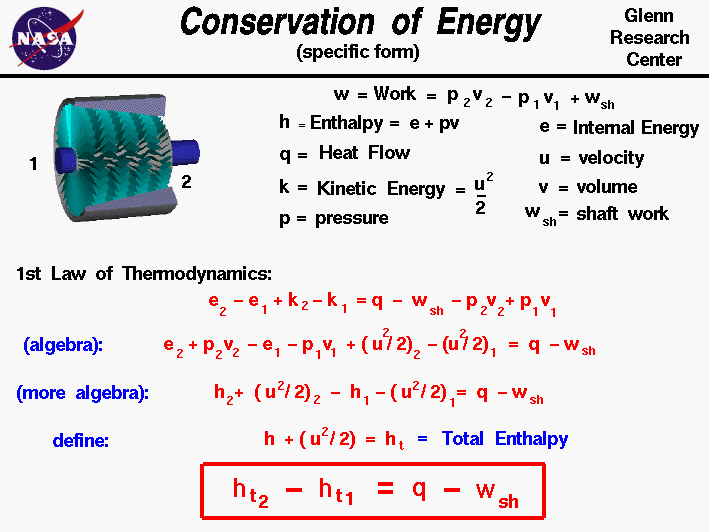
Conservation Of Energy

Mass And Energy Rate Balance Equations For Bifstig Cycle Components Download Table

Thermodynamics Ebook Steady Flow Devices 1
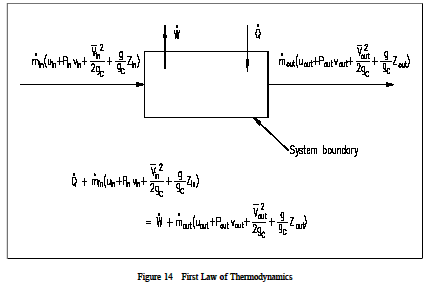
First Law Of Thermodynamics Engineers Edge Www Engineersedge Com

Mass And Energy Balances
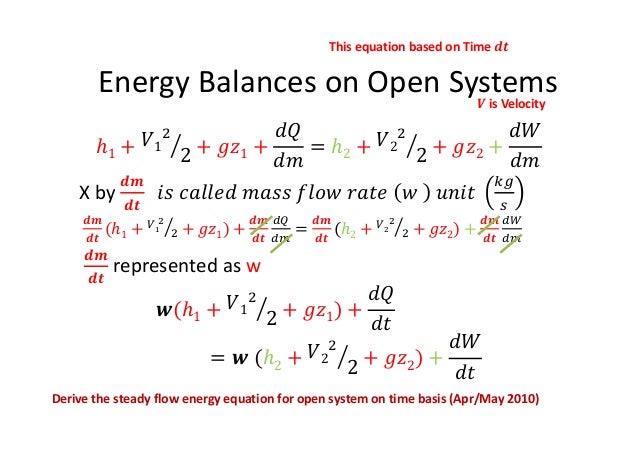
02 Part5 Energy Balance

Mass And Energy Balance Equations For Absorption Refrigeration System Download Table
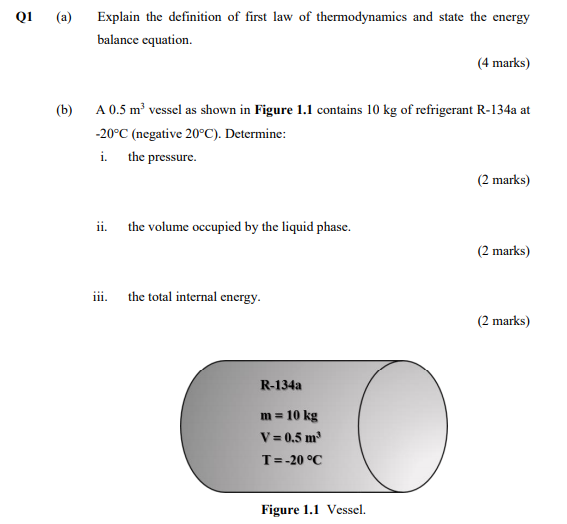
Solved Q1 A Explain The Definition Of First Law Of Ther Chegg Com

Mass And Energy Balances
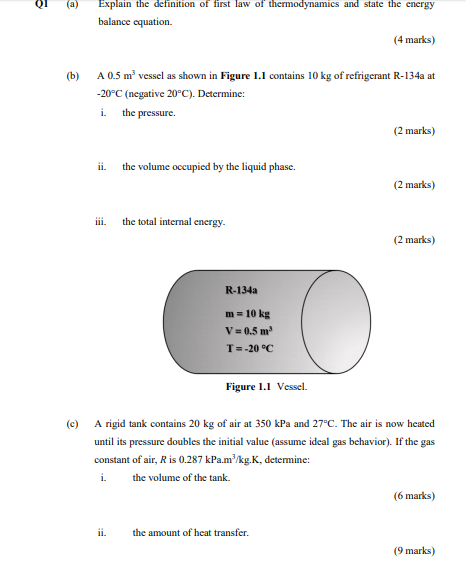
Solved Q1 A Explain The Definition Of First Law Of Ther Chegg Com

The First Law Of Thermodynamics Energy Balance For Unsteady Flow Processes Hydraulics And Pneumatics
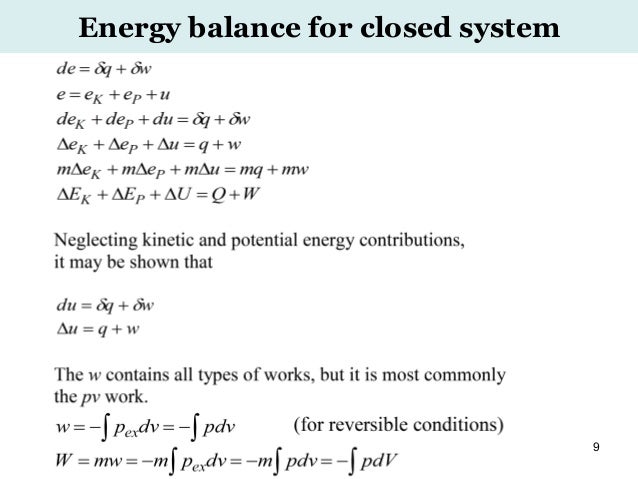
Advanced Chemical Engineering Thermodynamics 31 July 16
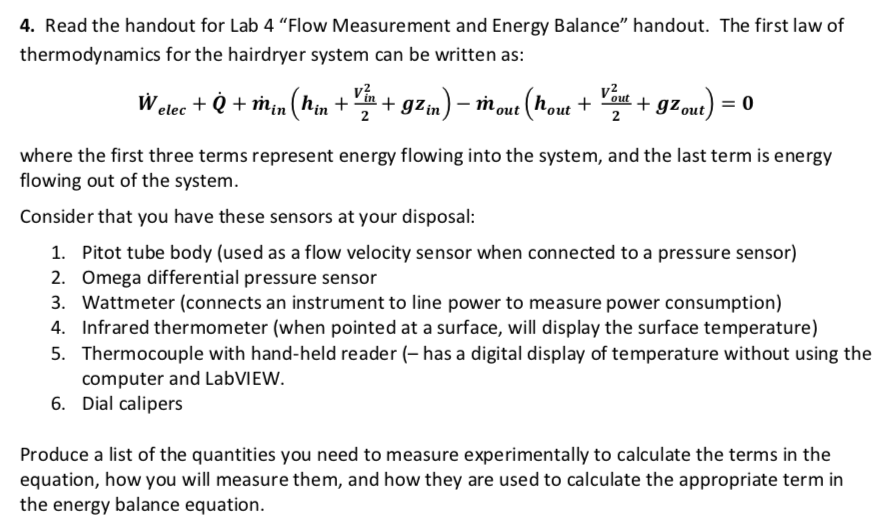
Solved 4 Read The Handout For Lab 4 Flow Measurement An Chegg Com
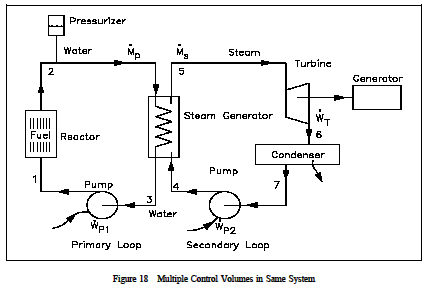
First Law Of Thermodynamics Engineers Edge Www Engineersedge Com

Thermodynamics Ebook Unsteady Flow Process

Thermodynamics Lecture 12 Control Volume Energy Balance Youtube
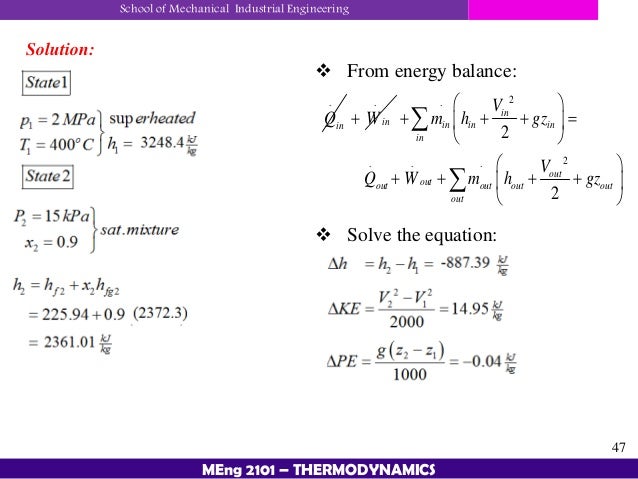
Chapter 4 First Law Of Thermodynamics Thermodynamics 1

Balance Equations And Laws Of Thermodynamics Thermodynamics Entropy
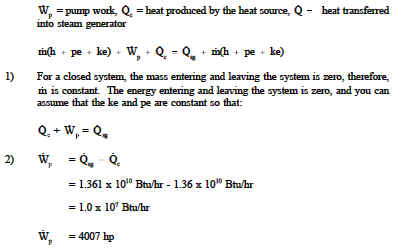
First Law Of Thermodynamics Engineers Edge Www Engineersedge Com
Mycourses lto Fi Mod Resource View Php Id 1485

Thermodynamics Ebook Entropy Balance For Control Volume

Chapter 3a The First Law Closed Systems Energy Updated 1 17 11

First Law Of Thermodynamics Engineers Edge Www Engineersedge Com

Thermodynamics Steady Flow Energy Balance 1st Law Throttle Youtube
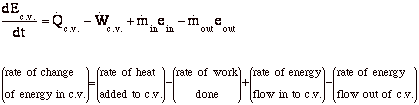
New Page 1



