In Vitro Testing Of Biomaterials
With recent studies suggesting poor correlation between current in vitro and in vivo testing of biomaterials, in vitro immune response assays may be more relevant and enhance ability in predicting acceptance prior to in vivo application Uptake of in vitro immune response assessment will allow for substantial reductions in experimental time and.
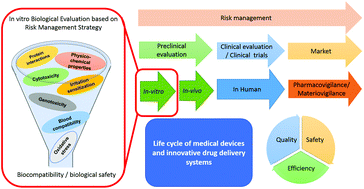
In vitro testing of biomaterials. Biocompatibility of polymerbased biomaterials and medical devices regulations, in vitro screening and riskmanagement Mélisande Bernard ParisSud University, Faculty of Pharmacy, EA 401, "Groupe Matériaux et Santé", Paris, France emilejubeli@upsudfr. Unfortunately, cytotoxicity testing most commonly uses fibroblasts in vitro for a short period of time, commonly up to 7 days, and then claims are usually made that the biomaterial is noncytotoxic Rarely do biomaterial scientists consider that cell lines are tumor derived and therefore do not necessarily represent the specific cells and. In Vivo Biocompatibility Testing Once in vitro testing has been completed, in vivo biological testing is performed, with the extent of type of testing to be performed based upon the device’s intended use In vivo testing can range from skin irritation testing, to sensitization testing, implantation testing and systemic toxicity testing Turnaround time for tests can range from three weeks to greater than several months, depending on the specific test data needed.
The Role of In Vitro Immune Response Assessment for Biomaterials 1 Introduction Grafting is required to improve healing and restore tissue integrity and is used in cases where the 2 Current In Vitro Testing of Biomaterials The standard in vitro testing protocols for assessing novel. Besides the setting time and biocompatibility, in comprehensive in vitro studies, it is found that the release of Mg 2 ions in CPC enhances the activity of osteoblast differentiation and inhibits osteoclast formation, which is an important aspect that should be taken into account for a biomaterial. In vitro cell testing of degradable bioceramics such as brushite or monetite is often challenging due to the ion release into or adsorption from the culture medium These ionic changes are then mostly responsible for cell proliferation and activity, which prohibits the investigation of effects originating from surface topography or further material modifications.
Vitro and in vivo outcomes of biomaterials tailored for bone in a European multicentre study between 8 universities that included 36 independent in vivo studies and 47 individual in vitro assays testing 93 biomaterial variables The focus of this study is on the correlation between in vitro testing and early stage (ie small animal) in vivo models. Functionalizing biomaterials with peptides or polymers that enhance recruitment of endothelial cells (ECs) can reduce blood coagulation and thrombosis To assess endothelialization of materials in vitro, primary ECs are generally used, although the characteristics of these cells vary among the donors and change with time in culture. American Preclinical Services offers multiple invitro methodologies that can be used for the evaluation of biocompatibility, toxicology and lotrelease testing Our invitro services include tests that meet ISO , USP , and JMHLW guidelines so you can rest assured that our tests will accommodate the necessary regulatory requirements We are proud to offer alternative methods for the.
During the last few years, on the basis of their physicochemical characteristics, thermoplastic materials, already used in several advanced industries, have become very attractive candidates for biomedical applications as matrix for composite femoral stems and bone plates In the present study, the biocompatibility of a thermoplastic material, polyetherimide (PEI), was investigated both in. Keywords zeta potential, biomaterials, in vitro testing, surface charge, reactivity, protein absorption Citation Ferraris S, Cazzola M, Peretti V, Stella B and Spriano S (18) Zeta Potential Measurements on Solid Surfaces for in Vitro Biomaterials Testing Surface Charge, Reactivity Upon Contact With Fluids and Protein Absorption Front. Biomimetic materials are designed to stimulate specific cellular responses at the molecular level To improve the soundness of in vitro testing of the biological impact of new materials, appropriate cell systems and technologies must be standardized also taking regulatory issues into consideration In this study, the biological and molecular effects of different scaffolds on three neural.
The Biomaterials Engineering and Testing (BET) Core provides support for the synthesis, characterization, in vitro testing, and analysis of biomaterials and bioengineered systems as well as their preparation for in vivo testing under the Clemson Translational Research and Imaging (CTRIC) Core. Moreover, Znbased biomaterials promoted stem cell differentiation to induce the extracellular matrix mineralization process In addition, in vivo animal testing using subcutaneous, bone, and vascular implantations revealed that the acute toxicity and immune response of Znbased biomaterials were minimal/moderate, comparable to that of AZ31. In vivo testing is important for biomaterials testing while in vitro tests cannot replace them, but only can be complementary Material and methods In our study, the implant compatibility testing.
A review of in vitro cell culture testing methods for bioactive glasses and other biomaterials for hard tissue regeneration E Jablonská, D Horkavcová, D Rohanová and D S Brauer, J Mater. The Laboratory for “in vitro” testing of biomaterials provides a working structure that may provide valid results in the biocompatibility evaluation of materials and medical devices Biocompatibility is defined as compatibility with a living tissue or a living system by lack of toxicity, injury or physiological reactivity and without. Besides the setting time and biocompatibility, in comprehensive in vitro studies, it is found that the release of Mg 2 ions in CPC enhances the activity of osteoblast differentiation and inhibits osteoclast formation, which is an important aspect that should be taken into account for a biomaterial.
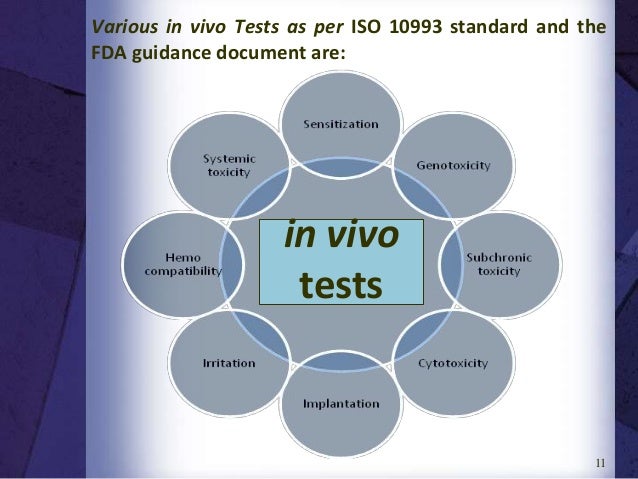
Extractable leachable testing, or chemical characterization, is also usually conducted before the in vivo testing Cytotoxicity, chemical characterization and in vivo biocompatibility testing is described below Cytotoxicity In Vitro – ISO Cytotoxicity is a biocompatibility test performed on mammalian cells in culture. A biomaterial is required not only to be nontoxic but it should also allow the cells in contact with it to benefit from the interaction The testing of all these functions is quite difficult in vivo but can be studied in detail with a sensitive panel of in vitro tests. Therefore, the testing of polymers in animals as well as in the test tube is a ENDDTHELIUM requirement for all materials prior to implantation in humans Fibrous capsule formation in v&w and toxicity/proliferation Endothelial cells line the blood vessels of arteries, veins and assays in vitro provide the basis for one parameter of caprllaries.
More recently, microfluidic organotypic patientspecific cancer models of multiple myeloma have been developed to test treatment effectiveness in vitro 56–59 56 S Bersini et al, “ A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone,” Biomaterials 35, 2454– 2461 (14). Here, the status of in vitro thrombogenicity testing methods for biomaterials is reviewed,. Besides the setting time and biocompatibility, in comprehensive in vitro studies, it is found that the release of Mg 2 ions in CPC enhances the activity of osteoblast differentiation and inhibits osteoclast formation, which is an important aspect that should be taken into account for a biomaterial.
Abdominal hernia repair is a frequently performed surgical procedure worldwide Currently, the use of polypropylene (PP) surgical meshes for the repair of abdominal hernias constitutes the primary surgical approach, being widely accepted as superior to primary suture repair Surgical meshes act as a reinforcement for the weakened or damaged tissues and support tissue restoration. 30 P Thevenot, W Hu and L Tang, Surface chemistry influences implant biocompatibility, Curr Top Med Chem 8, 270 (08) Google Scholar Crossref;. Specimens made of three different biodegradable materials (BDF 1, BDF 2 and BDF 3) were exposed invitro to an enzymecocktail under mechanical stress for 1, 2 and 3 weeks Degradation was determined by measuring dry weight loss, mechanical properties (compression test) and SEM surface analysis.
Using fresh human blood and adequate in vitro models, the hemocompatibility of bloodcontacting biomaterials can be studied accurately Compared to in vivo animal models, in vitro models allow the analysis under well controllable conditions such as blood flow, anticoagulation and eliminate disturbing factors related to flow obstruction, surgery, and tissue effects ( van Oeveren, 13 ). 6 In vivo and in vitro testing for the biological safety evaluation of biomaterials and medical devices 61 Introduction Like all products intended to be used in humans, medical devices, and/or the materials they are 62 Pretesting considerations The evaluation of the biological safety. In this study, we showed that the effect of biomaterials on both neuron viability and differentiation is strongly influenced by the cellular system used for the test We also showed that cellbased HCS technology can be applied to biomaterial in vitro testing, thus improving robustness and statistical consistency of the results.
2/28/06 4 Question What is meant by the term “animal model”?. Abstract The association of in vitro tests and in vivo bone implants, has significantly improved the characterization of biomaterials for orthopaedic devices before their clinical use However, neither cell cultures nor most animals models used for these tests entirely reflect the clinical conditions in which biomaterials are implanted. In vitro thrombogenicity testing of biomaterials is reviewed regarding prerequisites for a reproducible in vitro testing, applied test systems, and test parameters for the characterization of the.
Abdominal hernia repair is a frequently performed surgical procedure worldwide Currently, the use of polypropylene (PP) surgical meshes for the repair of abdominal hernias constitutes the primary surgical approach, being widely accepted as superior to primary suture repair Surgical meshes act as a reinforcement for the weakened or damaged tissues and support tissue restoration. In vitro hemocompatibility testing The importance of fresh blood Biointerphases 11, (16);. Bovine serum is the lubricant recommended by several international standards for the wear testing of orthopaedic biomaterials;.
Biocompatibility of polymerbased biomaterials and medical devices regulations, in vitro screening and riskmanagement Mélisande Bernard ParisSud University, Faculty of Pharmacy, EA 401, "Groupe Matériaux et Santé", Paris, France emilejubeli@upsudfr. Despite a highly regulated environment, biocompatibility evaluation of biomaterials for medical devices is a complex task related to various factors that include mainly chemical nature and physical properties of the material, the contact tissue and duration of contact. Functionalizing biomaterials with peptides or polymers that enhance recruitment of endothelial cells (ECs) can reduce blood coagulation and thrombosis To assess endothelialization of materials in vitro, primary ECs are generally used, although the characteristics of these cells vary among the donors and change with time in culture Recently, primary cell lines immortalized by transduction of.
Implant studies are used to determine the biocompatibility of medical devices or biomaterials that directly contact living tissue other than skin (eg sutures, surgical ligating clips, implantable devices, etc) These tests can evaluate devices, which, in clinical use, are intended to be implanted for either shortterm or longterm periods. / Thrombogenicity and hemocompatibility of biomaterials Biointerphases 11, (16);. Moreover, Znbased biomaterials promoted stem cell differentiation to induce the extracellular matrix mineralization process In addition, in vivo animal testing using subcutaneous, bone, and vascular implantations revealed that the acute toxicity and immune response of Znbased biomaterials were minimal/moderate, comparable to that of AZ31.
Functionalizing biomaterials with peptides or polymers that enhance recruitment of endothelial cells (ECs) can reduce blood coagulation and thrombosis To assess endothelialization of materials in vitro, primary ECs are generally used, although the characteristics of these cells vary among the donor. A surprisingly poor correlation between in vitro and in vivo assessments of biomaterials was revealed indicating a clear need for further development of relevant in vitro assays There was no significant overall correlation between in vitro and in vivo outcome The mean in vitro scores revealed a trend of covariance to in vivo score with 58 % The inadequacies of the current in vitro assessments highlighted here further stress the need for the development of novel approaches to in vitro. Genotoxicity •in vivo genotoxicity tests are carried out if indicated by the chemistry and/or composition of the biomaterial or if in vitro test results indicate potential genotoxicity •Initially, at least three in vitro assays should be used and two of these assays should utilize mammalian cells •The initial in vitro assays should cover the three levels of genotoxic effects DNA destruction, Gene mutations, and Chromosomal aberrations.
However, there are issues over its use due to batch variation, degrada. Biomimetic materials are designed to stimulate specific cellular responses at the molecular level To improve the soundness of in vitrotesting of the biological impact of new materials, appropriate cell systems and technologies must be standardized also taking regulatory issues into consideration. In this study, we showed that the effect of biomaterials on both neuron viability and differentiation is strongly influenced by the cellular system used for the test We also showed that cellbased HCS technology can be applied to biomaterial in vitro testing, thus improving robustness and statistical consistency of the results.
In vitro genotoxicity testing of biomaterial Genotoxicity is a broad term that includes. In this study, we showed that the effect of biomaterials on both neuron viability and differentiation is strongly influenced by the cellular system used for the test We also showed that cellbased HCS technology can be applied to biomaterial in vitro testing, thus improving robustness and statistical consistency of the results. Costs For evaluation of biomaterials the hostilelike in vitro environments (closest to the respective clinical conditions) are desirable with control of chemical, biological, mechanical and other parameters 1 ,2 This is of importance in highthroughput screening (HTS), usually implemented as a large number of smaller test.
Biocompatibility is one of the main prerequisites for the clinical use of biomaterials Central to the testing of biocompatibility is the estimation of cytotoxicity, which can be assessed in vitro by using a variety of different target primary cells or cell lines The influence of toxic agents derived from biomaterials on cellular functions and cell viability can be characterised by reductions. U Seyfert, In vitro hemocompatibility testing of biomaterials according to the ISO –4, Biomol Eng 19, 91 (02) Google Scholar Crossref;. In case of InVitro, pins degraded from the interior to exterior of the implants 8 INTRODUCTION • Protocol for biomaterial test provide by –American Society for Testing Material (ASTM) – International Standards Organization (ISO) – Government agencies, eg, the FDA 9 PART 2 Biological Testing of biomaterials 10.
Functionalizing biomaterials with peptides or polymers that enhance recruitment of endothelial cells (ECs) can reduce blood coagulation and thrombosis To assess endothelialization of materials in vitro, primary ECs are generally used, although the characteristics of these cells vary among the donors and change with time in culture. The identification of such carcinogenic effects of biomaterials using relevant in vitro approaches is required to be included in the compatibility testing regime 71 A variety of metals used in orthopedic implants has been demonstrated to induce carcinogenesis in vivo, especially particulates of cadmium, cobalt, cobalt–chromium alloy and. / In vitro blood flow model with physiological wall shear stress for hemocompatibility testing—An example of coronary.
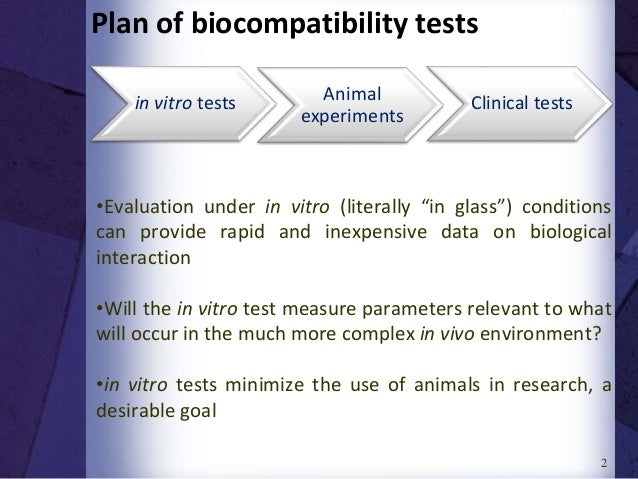
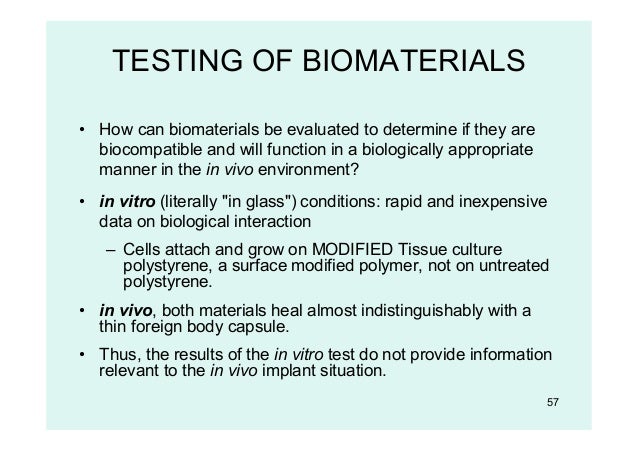
BIOCOMPATIBILITY TEST Evaluation under in vitro (literally "in glass") conditions Rapid and inexpensive data Evaluation of biomaterials by method that use isolated, adherent cells in culture to measure cytotoxicity and biological compatibility Use of tissue culture/bacteria on Petri dish Animals are used for testing of biomaterials to model. Biological Testing of Biomaterials 2/28/06 2 Question What do we mean by the term “in vitro?” 2/28/06 3 Question Will an in vitro test measure parameters that are relevant predictors of what will occur in the body (in vivo)?. The identification of such carcinogenic effects of biomaterials using relevant in vitro approaches is required to be included in the compatibility testing regime 71 A variety of metals used in orthopedic implants has been demonstrated to induce carcinogenesis in vivo, especially particulates of cadmium, cobalt, cobalt–chromium alloy and.
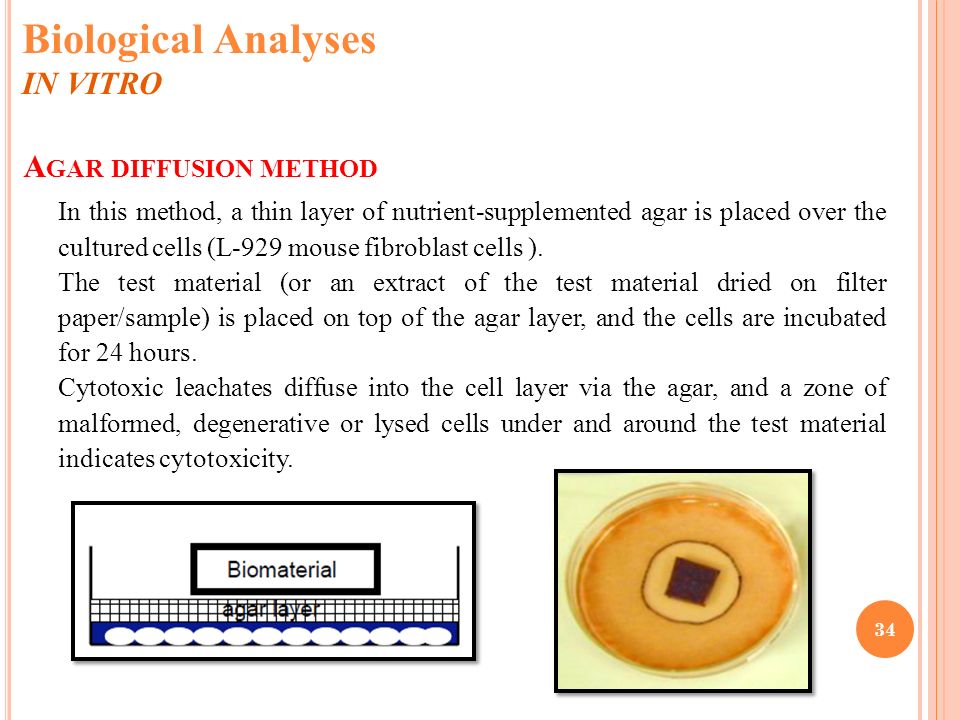
In vitro thrombogenicity testing of biomaterials is reviewed regarding prerequisites for a reproducible in vitro testing, applied test systems, and test parameters for the characterization of the. Besides the setting time and biocompatibility, in comprehensive in vitro studies, it is found that the release of Mg 2 ions in CPC enhances the activity of osteoblast differentiation and inhibits osteoclast formation, which is an important aspect that should be taken into account for a biomaterial. An in vitro method has been developed for screening of candidate biomaterials in an early phase of their development The test is based on L‐929 mouse fibroblast cultures and their response to powdered polymer samples.
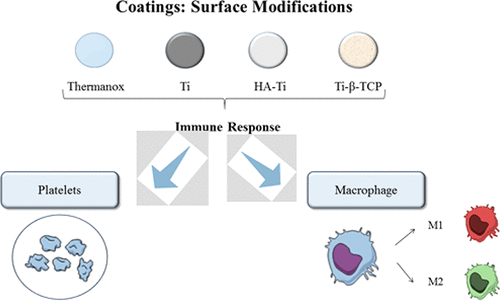
Biomaterial Property Effects On Platelets And Macrophages An In Vitro Study Acs Biomaterials Science Engineering X Mol

New Method Of Synthesis And In Vitro Studies Of A Porous Biomaterial Sciencedirect

Tissues Biomaterials Testing Solutions Instron
In Vitro Testing Of Biomaterials のギャラリー

1 Advantage And Disadvantage Of In Vivo And In Vitro Test Download Table

Buy In Vitro Testing Of Biomaterials Book Online At Low Prices In India In Vitro Testing Of Biomaterials Reviews Ratings Amazon In

Lecture 1 College ntekeningen 1 Studeersnel

In Vitro Thrombogenicity Testing Of Biomaterials Braune 19 Advanced Healthcare Materials Wiley Online Library

Biological Testing Of Biomaterials Biomaterial Toxicity

Biocompatibility Wikipedia

Comparison Of Modified Chandler Roller Pump And Ball Valve Circulation Models For In Vitro Testing In High Blood Flow Conditions Application In Thrombogenicity Testing Of Different Materials For Vascular Applications Topic
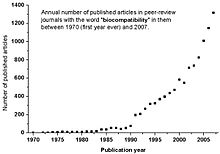
Biocompatibility Wikipedia

Ppt Testing Biomaterials Powerpoint Presentation Free Download Id

Ppt Testing Biomaterials Powerpoint Presentation Free Download Id

Biocompatibility Testing Of Biomaterials

In Vitro Testing Of Biomaterials For Neural Repair Focus On Cellular Systems And High Content Analysis Bioresearch Open Access
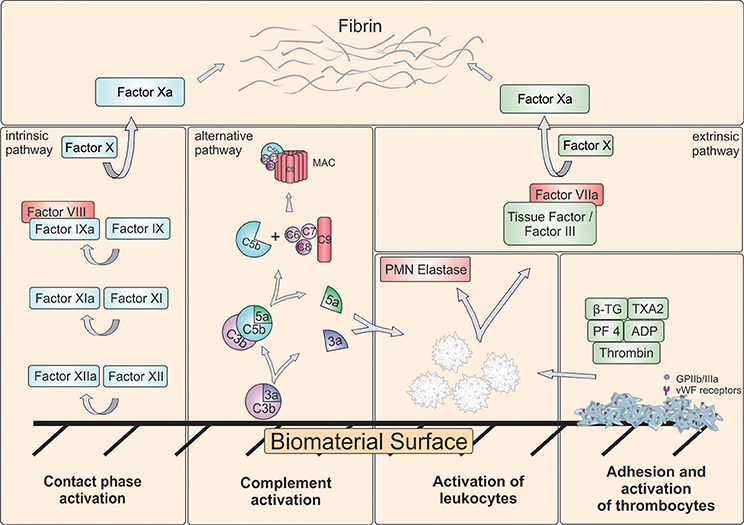
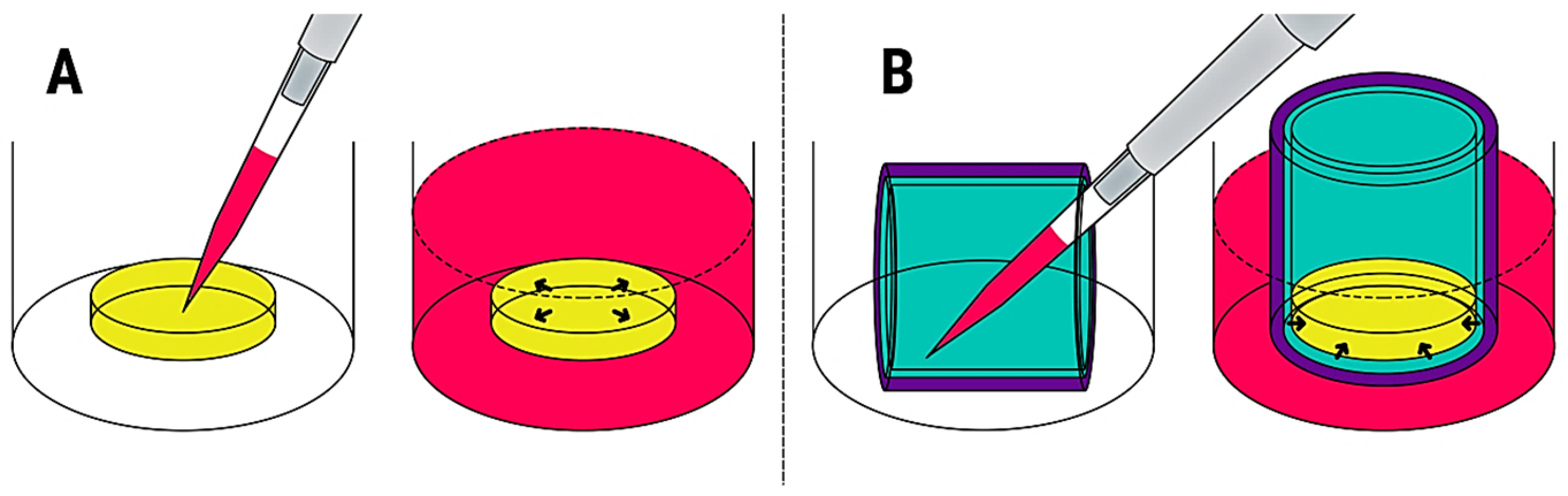
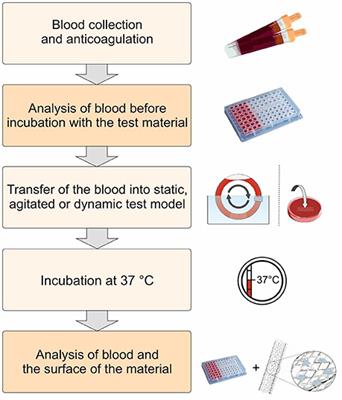
Frontiers Blood Contacting Biomaterials In Vitro Evaluation Of The Hemocompatibility Bioengineering And Biotechnology

Biocompatibility Testing Of Biomaterials
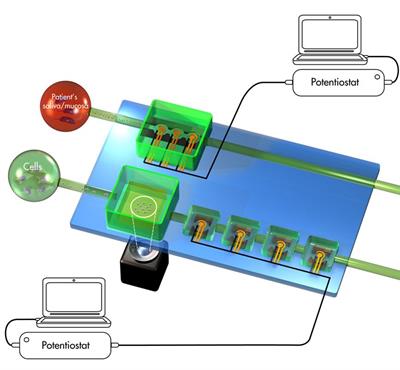
Adverse Reactions To Biomaterials State Of The Art In Biomaterial Risk Assessment Immunomodulation And In Vitro Models For Biomaterial Testing Frontiers Research Topic

Asaiojournal An In Vitro Blood Flow Loop System For Evaluating The Thrombogenicity Of Medical Devices And Biomaterials T Co Jkzs3fxqzj Us Fda Fda Medicaldevices Biomaterials Flowloop Hemocompatibility Thrombosis

Biocompatibility

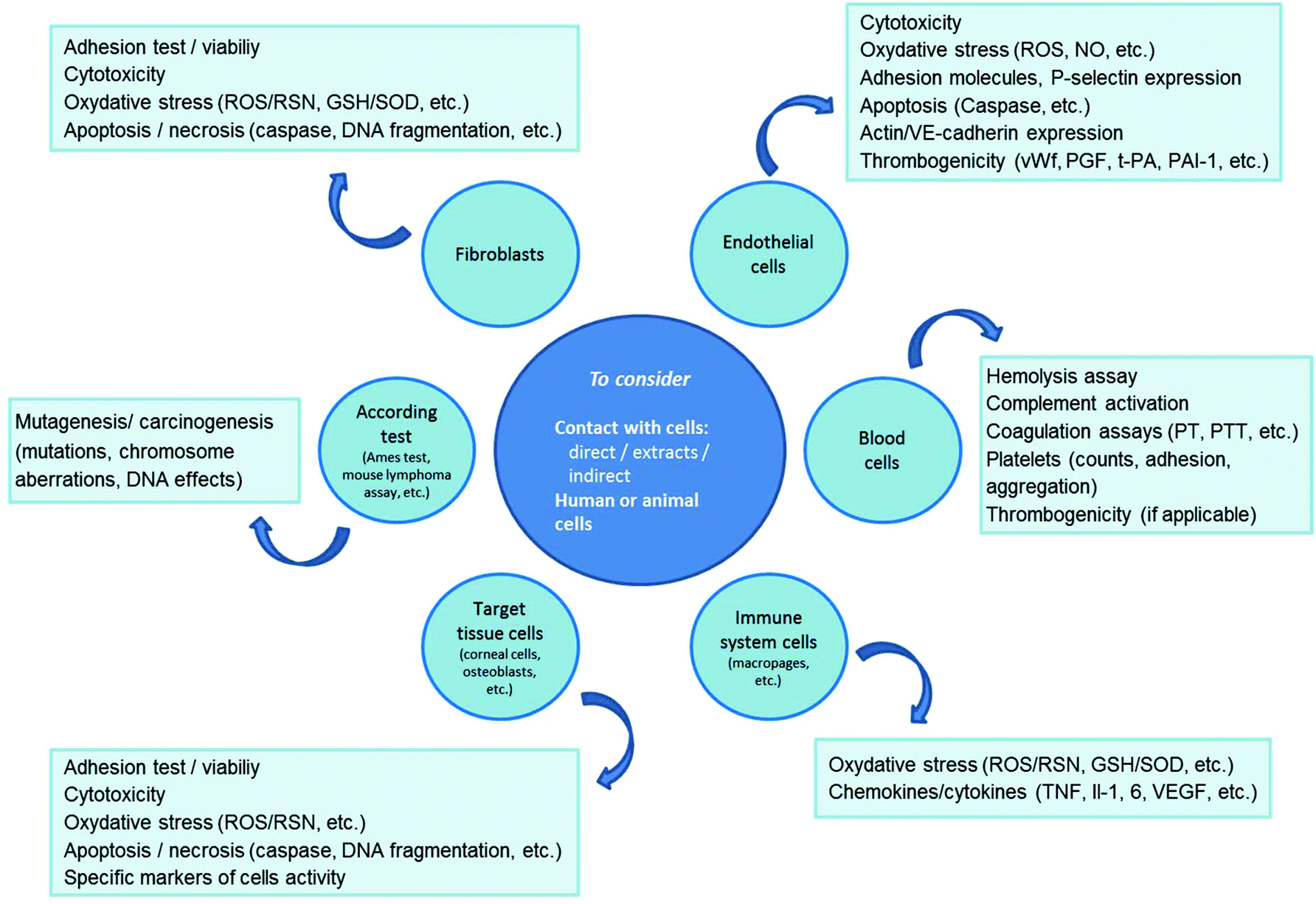
The Summary Of The Most Important Cell Biomaterial Interactions That Need To Be Considered During In Vitro Biocompatibility Testing Of Bone Scaffolds For Tissue Engineering Applications Sciencedirect

In Vivo And In Vitro Experiments For The Evaluation Of Porous Biomaterials Bentham Science

Biocompatibility Testing Of Biomaterials

Bioinformatics Based Selection Of A Model Cell Type For In Vitro Biomaterial Testing Semantic Scholar

Retrieval Analysis The Inappropriateness Of In Vitro Testing Protocols Orthodontic Biomaterials Network
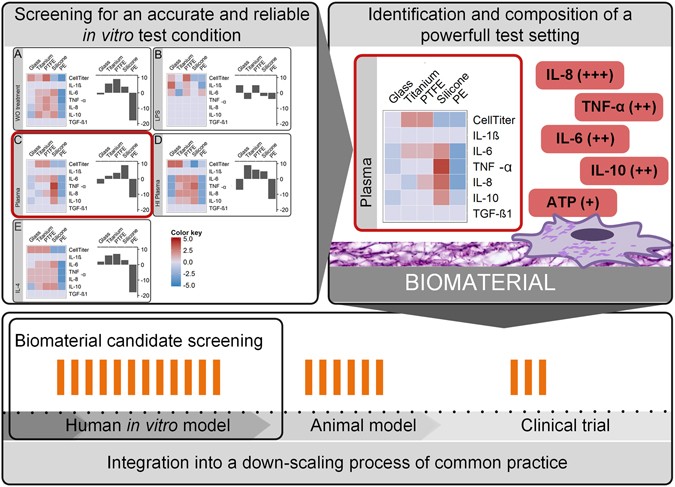
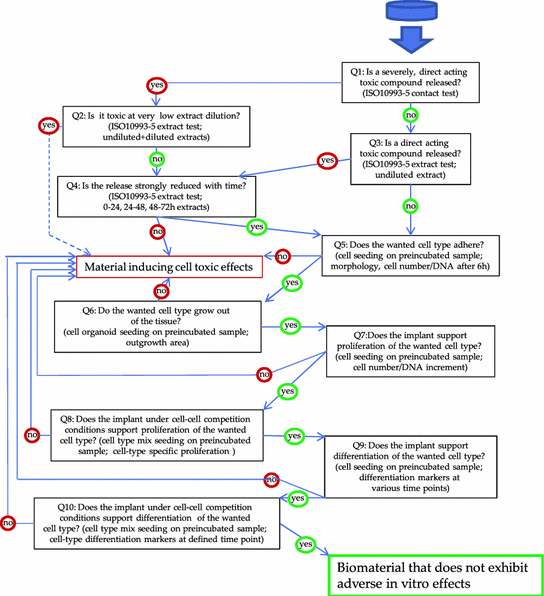
A Comparative Multi Parametric In Vitro Model Identifies The Power Of Test Conditions To Predict The Fibrotic Tendency Of A Biomaterial Scientific Reports
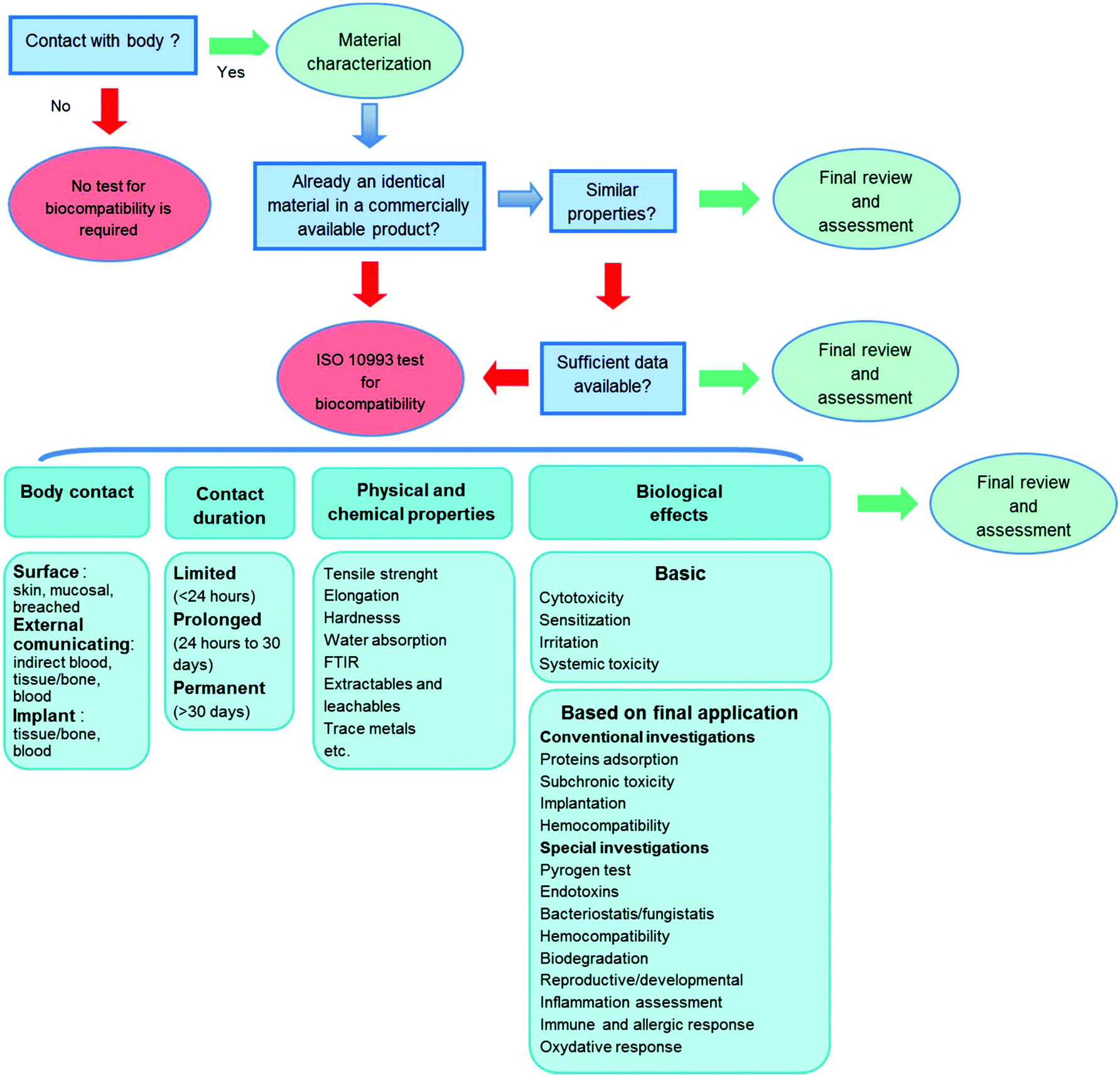
Biocompatibility Of Polymer Based Biomaterials And Medical Devices Regulations In Vitro Screening And Risk Management Biomaterials Science Rsc Publishing

1 Advantage And Disadvantage Of In Vivo And In Vitro Test Download Table
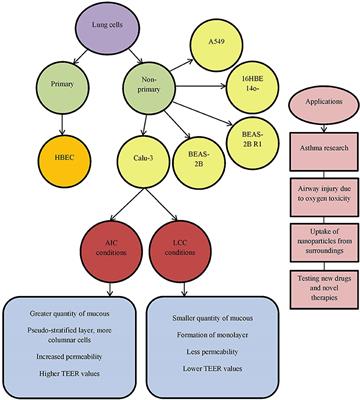
Frontiers In Vitro Models And On Chip Systems Biomaterial Interaction Studies With Tissues Generated Using Lung Epithelial And Liver Metabolic Cell Lines Bioengineering And Biotechnology

Bone Remodelling In Vitro Where Are We Headed A Review On The Current Understanding Of Physiological Bone Remodelling And Inflammation And The Strategies For Testing Biomaterials In Vitro Sciencedirect

Magnesium Biomaterials Design Testing And Best Practice Springerbriefs In Materials 13 Kirkland Nicholas Travis Birbilis Nick Amazon Com

Biocompatibility Testing Of Biomaterials
Biomaterials Springerlink
Biocompatibility Testing Of Biomaterials
Ppt Biomaterials An Introduction Powerpoint Presentation Free Download Id

In Vitro Thrombogenicity Testing Of Biomaterials Braune 19 Advanced Healthcare Materials Wiley Online Library

Handa Biomaterials Lab Hitesh Handa University Of Georgia
Biocompatibility Of Polymer Based Biomaterials And Medical Devices Regulations In Vitro Screening And Risk Management Biomaterials Science Rsc Publishing

Biocompatibility Testing Of Biomaterials

Orthopedic Biomaterials Bm3106 College Biomaterial Is Nonviable Material Used Studocu
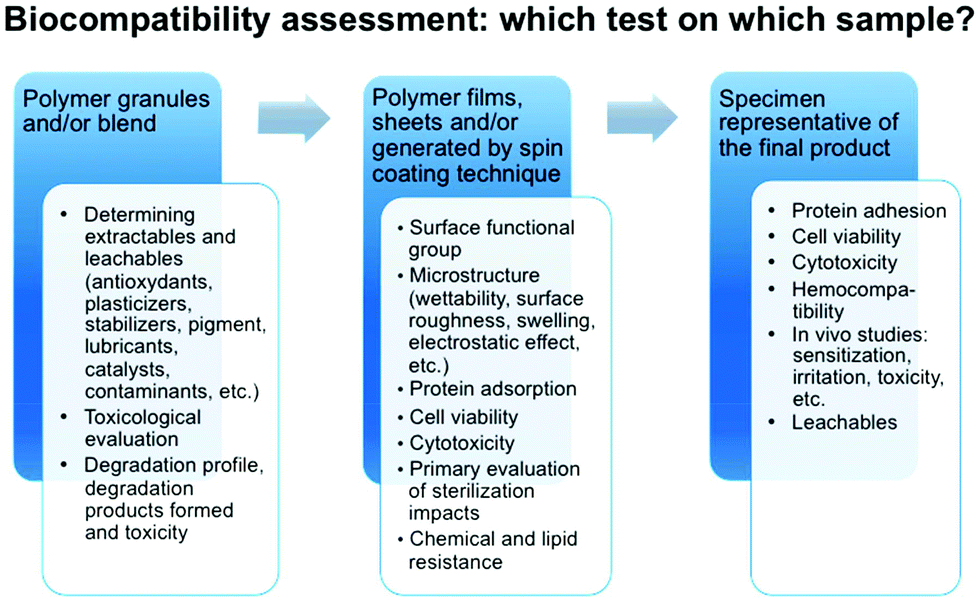
Biocompatibility Of Polymer Based Biomaterials And Medical Devices Regulations In Vitro Screening And Risk Management Biomaterials Science Rsc Publishing

Cells Free Full Text Biomimetic In Vitro Model Of Cell Infiltration Into Skin Scaffolds For Pre Screening And Testing Of Biomaterial Based Therapies
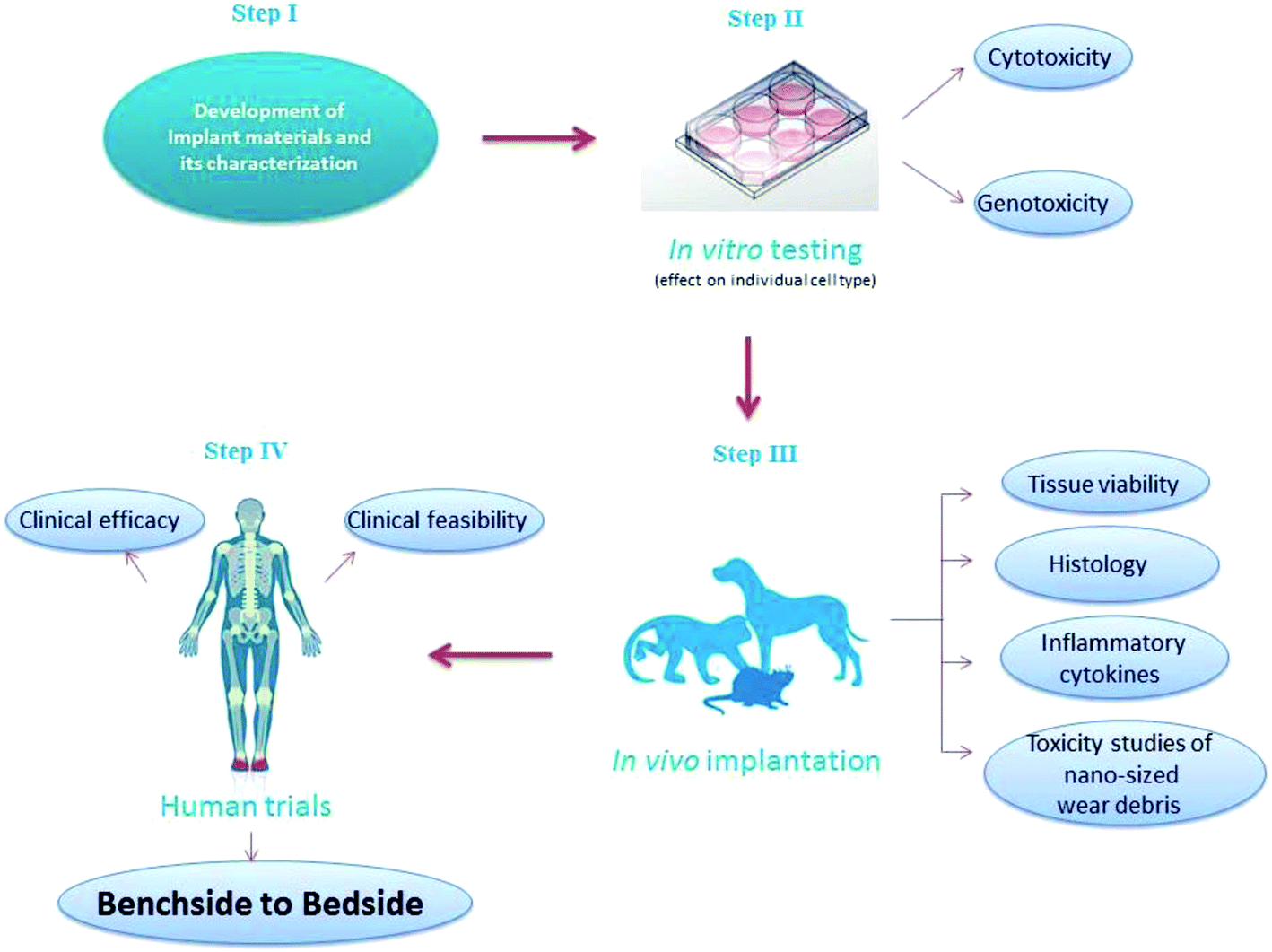
In Vitro In Vivo Assessment And Mechanisms Of Toxicity Of Bioceramic Materials And Its Wear Particulates Rsc Advances Rsc Publishing Doi 10 1039 C3ra444j
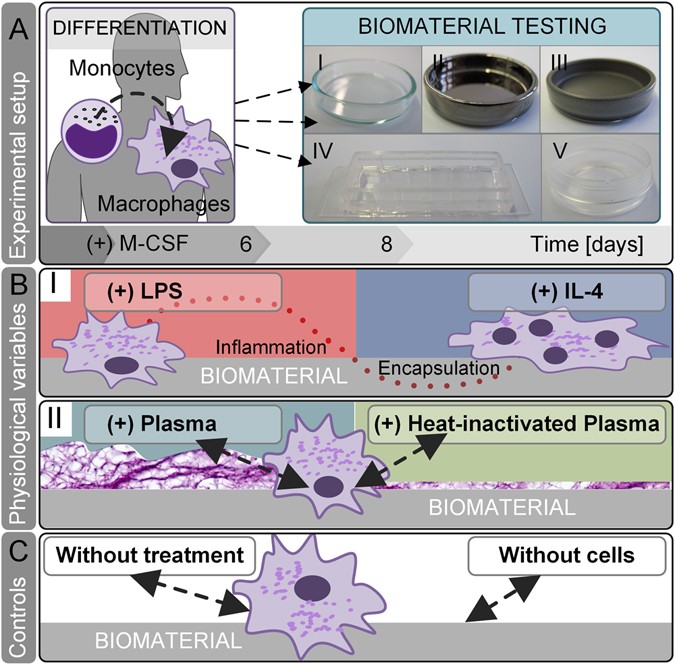
A Comparative Multi Parametric In Vitro Model Identifies The Power Of Test Conditions To Predict The Fibrotic Tendency Of A Biomaterial Scientific Reports
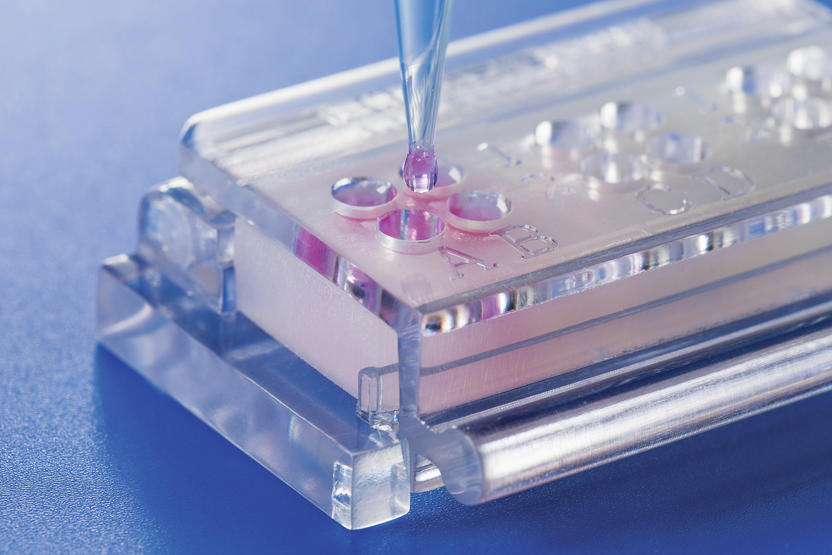
Biomaterial In Vitro Testing 2 0 Standardized Quantitative Resource Saving An Innovative In Vitro Test System For Quantitative Testing Methods In Direct Cell Material Contact
Plos One In Vitro Endothelialization Test Of Biomaterials Using Immortalized Endothelial Cells
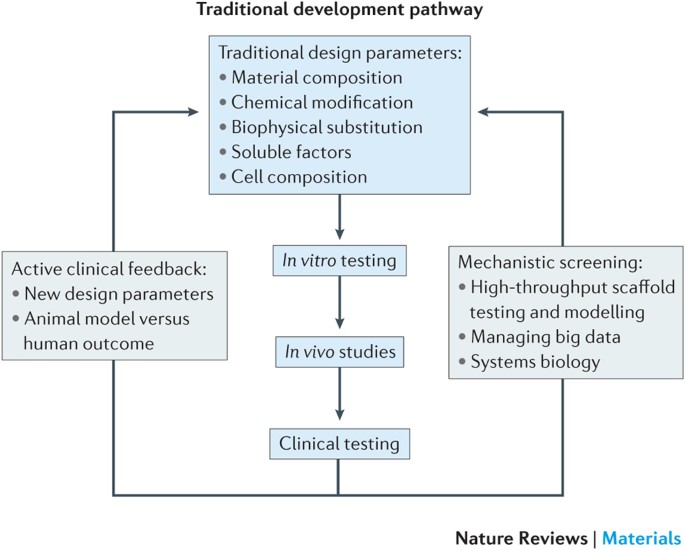
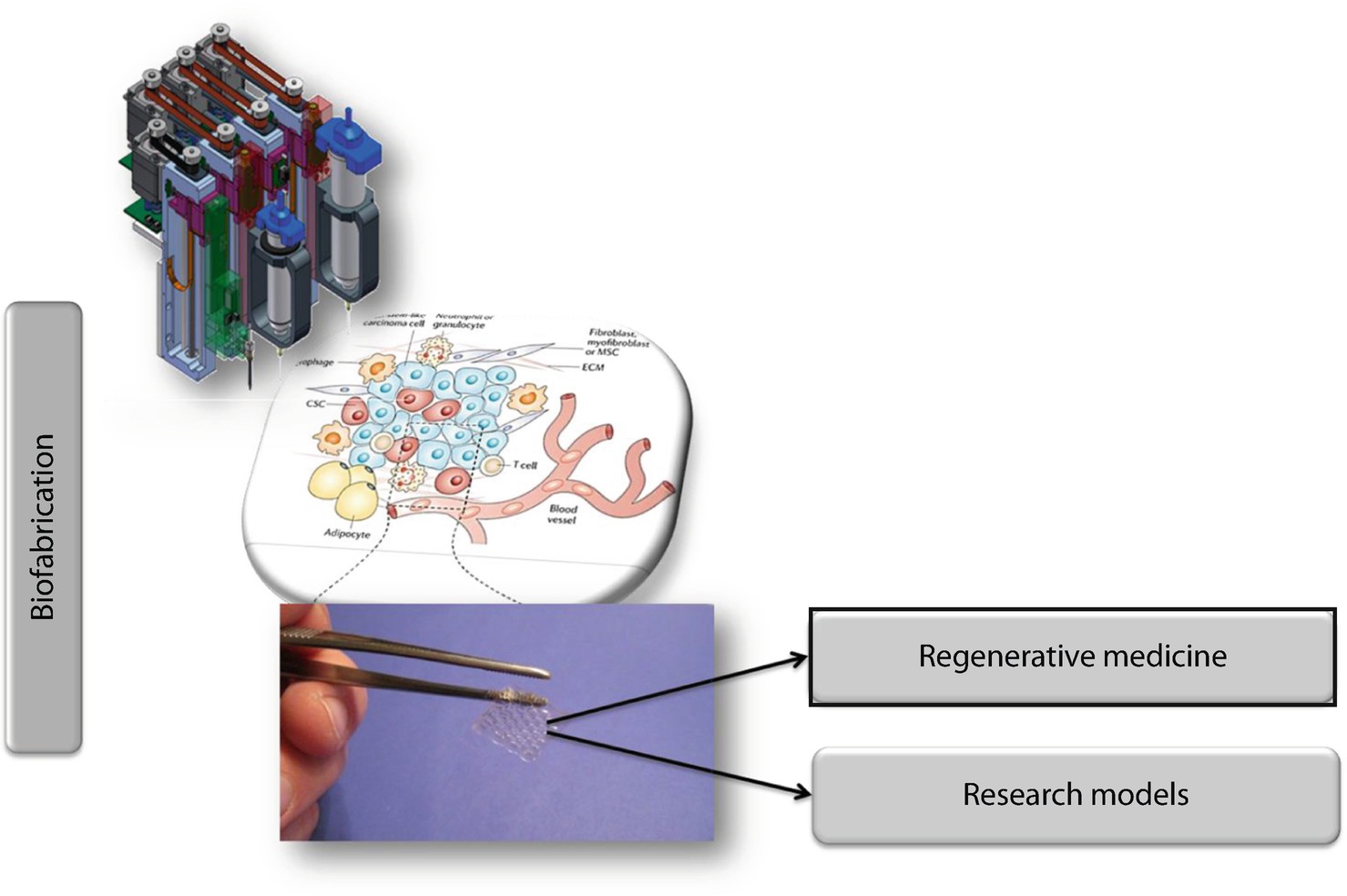
Design Clinical Translation And Immunological Response Of Biomaterials In Regenerative Medicine Nature Reviews Materials
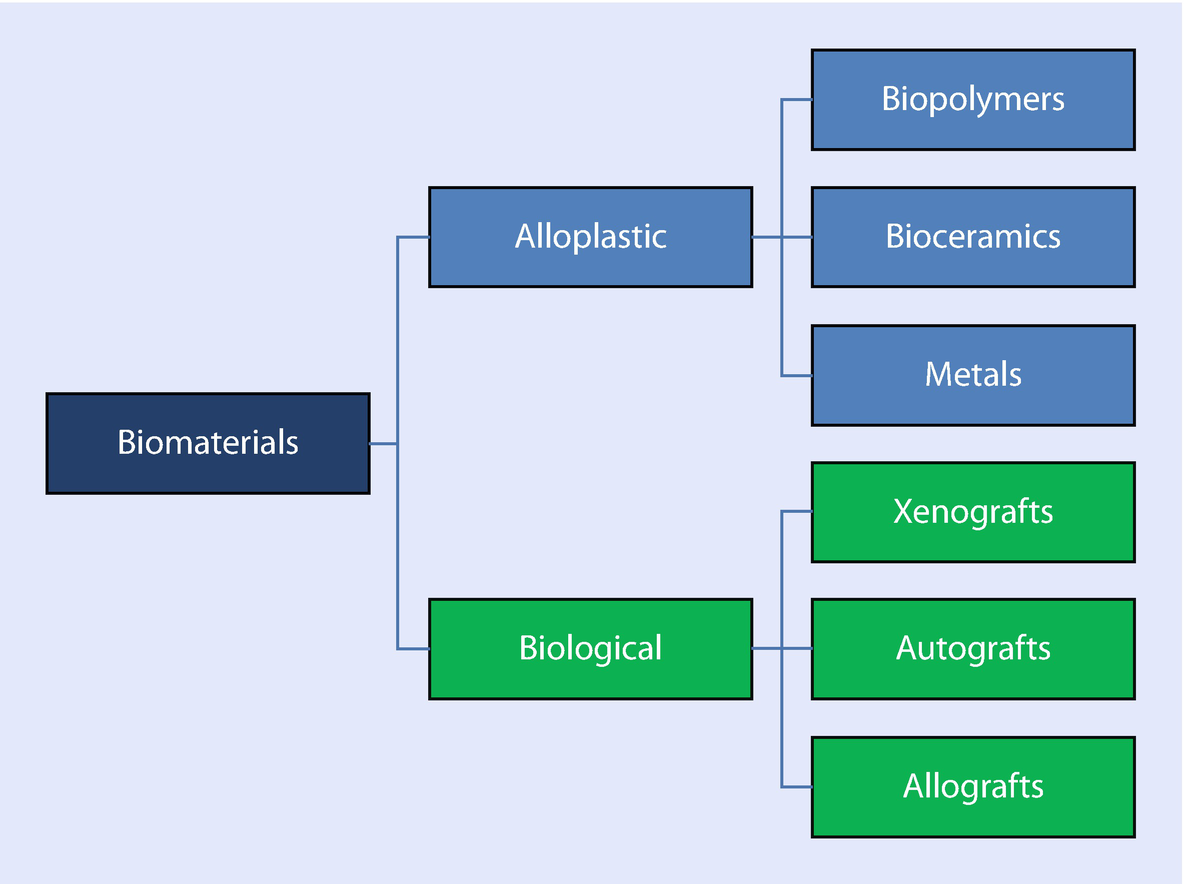
Biomaterials Springerlink

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

Biomaterials Laboratory Research Groups

Pdf Biocompatibility Study Of Polymeric Biomaterials Sonia Maria Malmonge Academia Edu
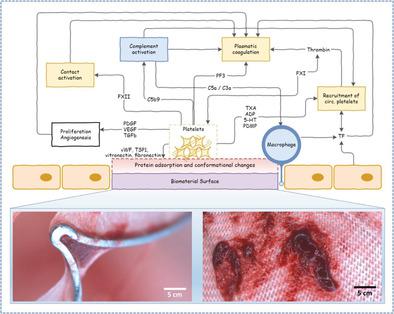
In Vitro Thrombogenicity Testing Of Biomaterials Advanced Healthcare Materials X Mol
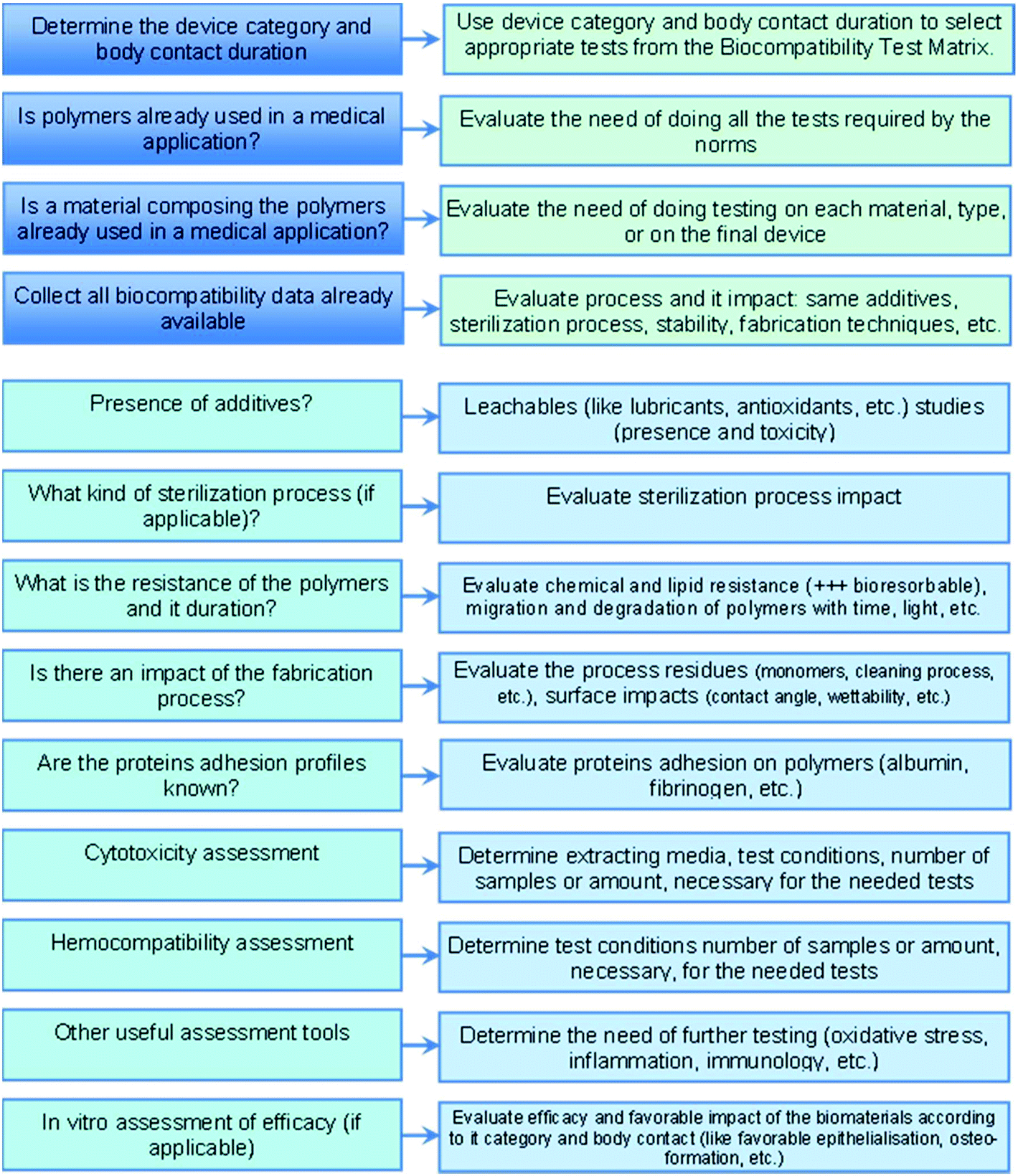
Biocompatibility Of Polymer Based Biomaterials And Medical Devices Regulations In Vitro Screening And Risk Management Biomaterials Science Rsc Publishing

Pdf A Surprisingly Poor Correlation Between In Vitro And In Vivo Testing Of Biomaterials For Bone Regeneration Results Of A Multicentre Analysis Semantic Scholar
Biocompatibility Of Polymer Based Biomaterials And Medical Devices Regulations In Vitro Screening And Risk Management Biomaterials Science Rsc Publishing
Ppt Bme Experiment 10 Summer Ming Long Yeh 8 25 10 Powerpoint Presentation Id

Biocompatibility Testing Of Biomaterials
Evaluation Of Biocompatibility Using In Vitro Methods Interpretation And Limitations Springerlink

Biological Materials Selection Guide Engineering360

Biomaterials The b Is An Integral Part Of The Cns This Is A Great Review Highlighting The Recent Advances In Development Of In Vitro b Models For Fundamental Studies And

Figure 2 From Permeability Testing Of Biomaterial Membranes Semantic Scholar
Plos One In Vitro Endothelialization Test Of Biomaterials Using Immortalized Endothelial Cells

Lecture 8 Testing Of Biomaterials Bioceramic Biomaterial

3 A Biomaterials Engineer Has Synthesized A New M Chegg Com
Plos One In Vitro Endothelialization Test Of Biomaterials Using Immortalized Endothelial Cells
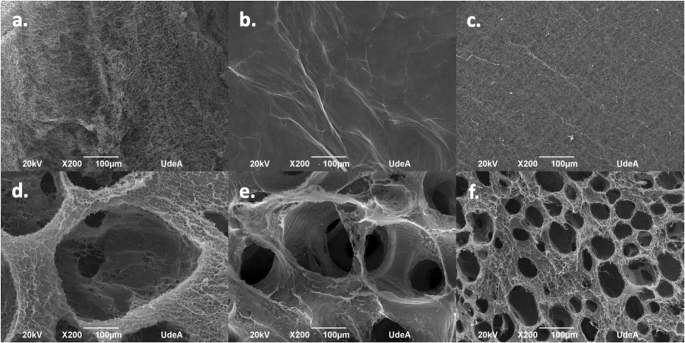
Ex Vivo And In Vivo Biocompatibility Assessment Blood And Tissue Of Three Dimensional Bacterial Nanocellulose Biomaterials For Soft Tissue Implants Scientific Reports

Hemocompatibility An Overview Sciencedirect Topics
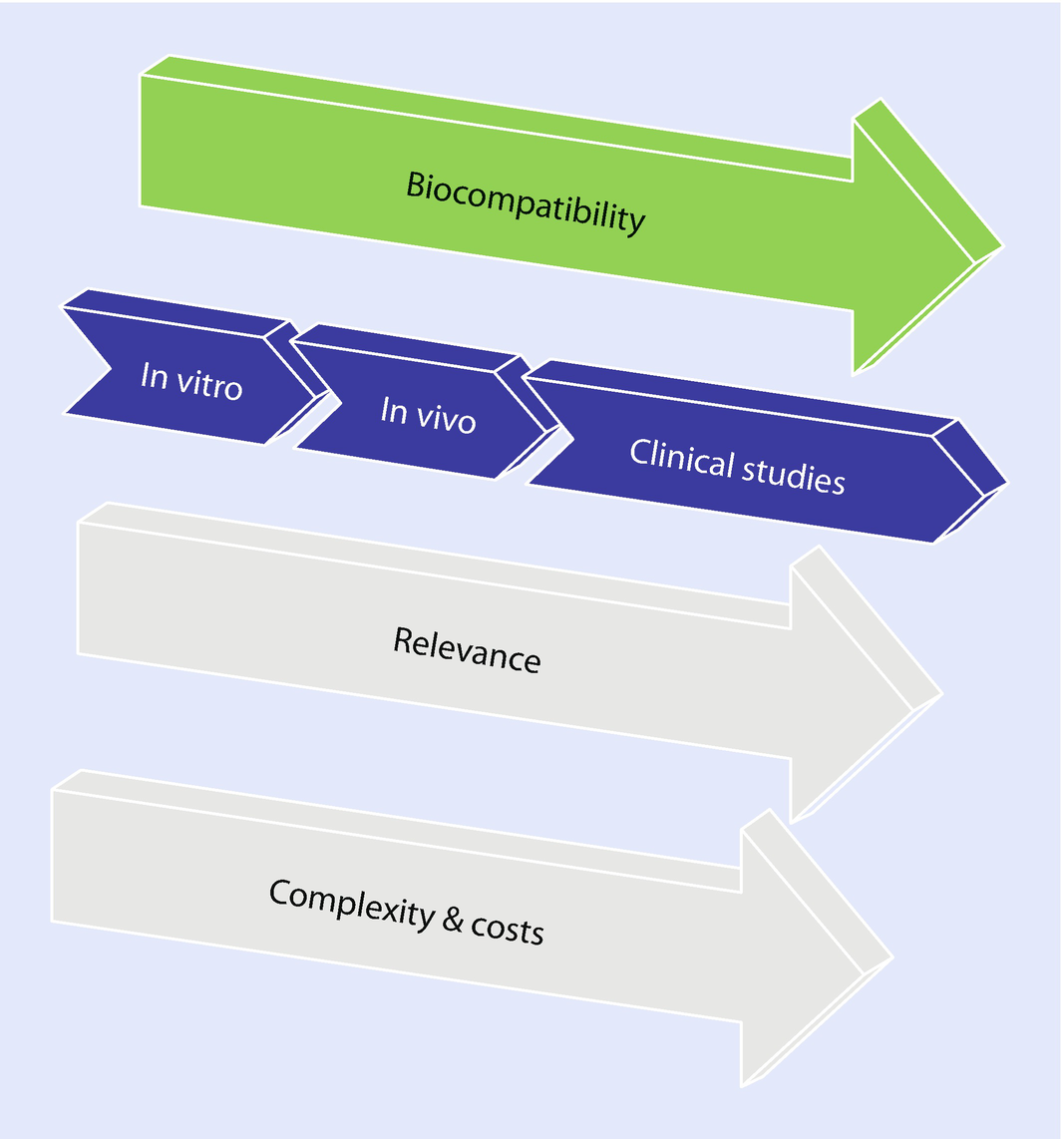
Biocompatibility Testing Of Biomaterials
Plos One In Vitro Endothelialization Test Of Biomaterials Using Immortalized Endothelial Cells

In Vivo And In Vitro Testing For The Biological Safety Evaluation Of Biomaterials And Medical Devices Sciencedirect

Bme Experiment 10 Summer Ming Long Yeh 8 25 Ppt Download

Pdf A Surprisingly Poor Correlation Between In Vitro And In Vivo Testing Of Biomaterials For Bone Regeneration Results Of A Multicentre Analysis

Degradation Phenomena On Polymeric Biomaterials Springerprofessional De

Biological Testing Of Biomaterials From

In Vitro Thrombogenicity Testing Of Biomaterials Braune 19 Advanced Healthcare Materials Wiley Online Library

Biomaterials Laboratory At Michigan State University

Biological Characterization Of Biomaterials In Vitro Tests Bentham Science

In Vitro Models To Test Orthopedic Biomaterials In View Of Their Clinical Application In Osteoporotic Bone Semantic Scholar

Pdf Are There Sufficient Standards For The In Vitro Hemocompatibility Testing Of Biomaterials

Pdf In Vitro Thrombogenicity Testing Of Biomaterials

In Vitro In Vivo And Post Explantation Testing Of Glucose Detecting Biosensors Current Methods And Recommendations Abstract Europe Pmc

In Vivo In Vitro And Analytical Biocompatibility Testing Services
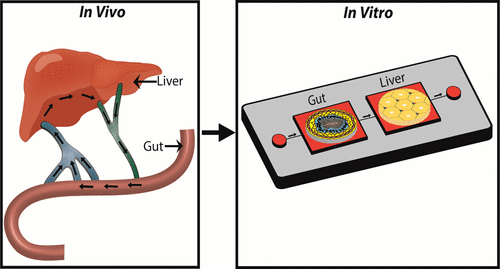
In Vitro Intestinal And Liver Models For Toxicity Testing Acs Biomaterials Science Engineering X Mol
Biomaterials Springerlink
Biomedical Engineering Biomaterials Ppt Download

Amazon Com Bio Tribocorrosion In Biomaterials And Medical Implants 13 Tribocorrosion In Artificial Joints In Vitro Testing And Clinical Implications Woodhead Publishing Series In Biomaterials Ebook Mathew M T Wimmer M A Kindle Store

In Vitro Biocompatibility Testing Of Bmp chen
Bit311 Biomaterials Unit 01 New

In Vitro Testing Of Biomaterials Toxicity And Biocompatibility Sciencedirect

Pdf Multiblock Copolyesters As Biomaterials In Vitro Biocompatibility Testing Bashar Saad Academia Edu
Frontiers Blood Contacting Biomaterials In Vitro Evaluation Of The Hemocompatibility Bioengineering And Biotechnology



