In Vitro Animal Testing
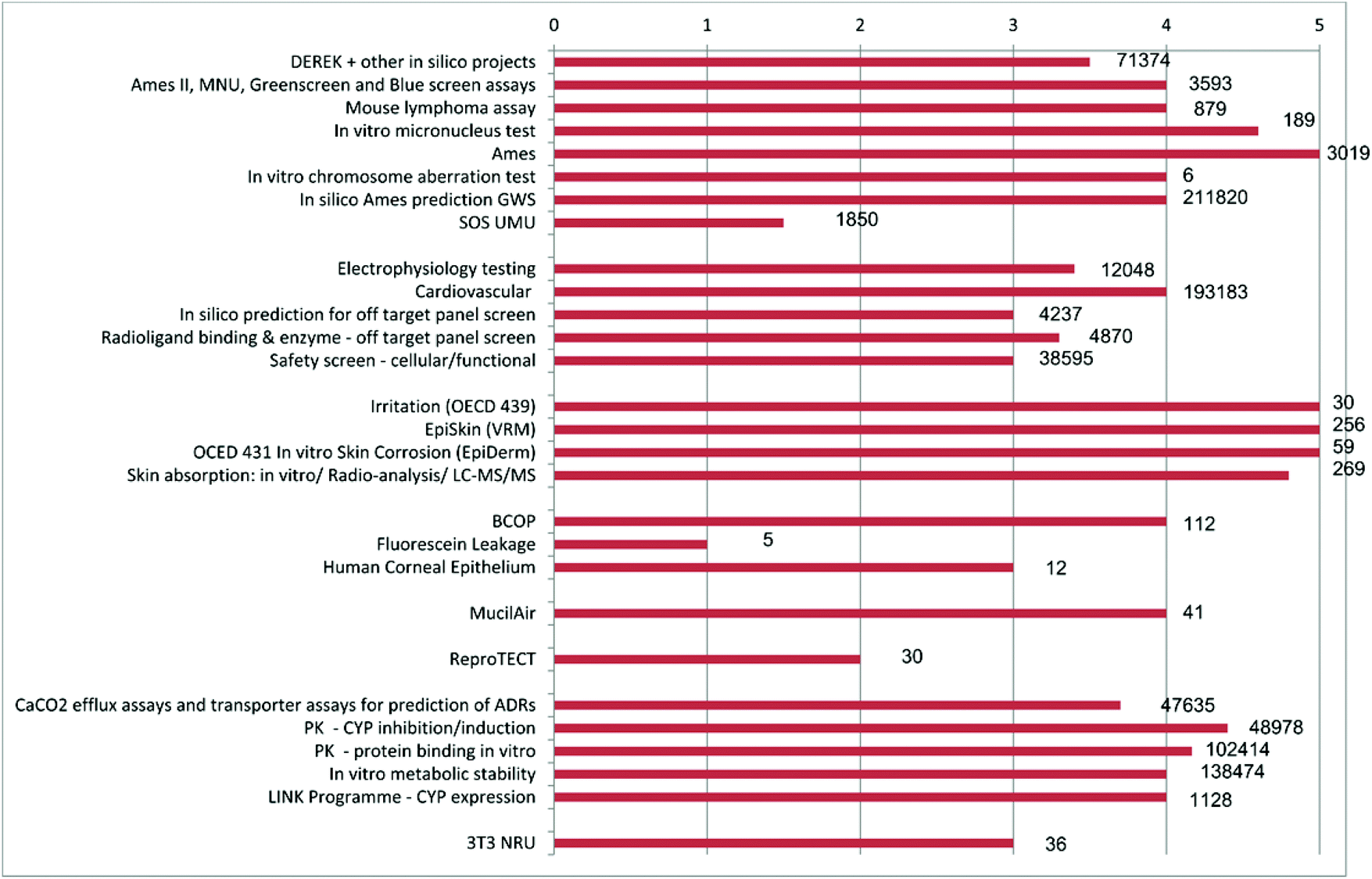
Indeed more than 70% of all in vitro assays captured by the survey were used since 10, peaking at just over 190,000 in 12 To be clear, reducing animal usage is not as simple as swapping out an animal assay for an in vitro alternative Alternatives can’t always tell us, for instance, if a compound will cause DNA damage leading to cancer, but they can some of the time, so we may be able to avoid using animals in the case of compounds with well understood adverse pathways.

In vitro animal testing. In vitro methods used in a laboratory can often include things like studying bacterial, animal, or human cells in culture Although this can provide a controlled environment for an experiment, it. In vitro studies, which are performed on cultured bacterial or mammalian cells, can be used as an initial screen to avoid the unnecessary use of animals in determining which candidates should move forward for further safety testing They are also used to replace many of the acute studies which have historically been performed in animals. On November 26, 19 In vitro comes from the Latin term "in glass" The term refers to studies of biological properties that are done in a test tube (ie in a glass vessel) rather than in a human or animal In vitro studies are often contrasted to in vivo ("in life") studies which are done inside an organism.
Unique among journals in its focus on in vitro biology in animals Offers studies of cellular, molecular, and developmental biology that employ or are relevant to organs, tissue, tumors, and cells in vitro Peerreviewed coverage of stateoftheart research since 1965 A journal of the Society for In Vitro Biology. In 1980, The New York Times featured a fullpage ad from an animal rights group, which lambasted a prominent cosmetics company for testing its products on the eyes of rabbitsThe campaign was so. In vitro testing for the assessment of your chemical is increasingly important as animal use continues to be replaced, refined and reduced Covance is helping lead innovation in this area so by partnering with us you will benefit from extensive experience in standard techniques and novel solutions.
In vitro is used to describe work that’s performed outside of a living organism This can include studying cells in culture or methods of testing the antibiotic sensitivity of bacteria. Other research methods such as in vitro testing (tests done on human cells or tissue in a petri dish) offer opportunities to reduce or replace animal testing 15 Technological advancements in 3D printing allow the possibility for tissue bioprinting a French company is working to bioprint a liver that can test the toxicity of a drug. Cells in culture are easier to molecularly manipulate, faster, cheaper and more reproducible than animal models Importantly, human cells can be studied in vitro and offer the potential of reducing animal use in several areas of study Many different kinds of cells are available to use in research, including established cell lines and stem cells Because stem cells have the ability to differentiate into many different types of cells, researchers are excited about their use as research models.
In summary, the data show a large increase and a continuing upwards trend in development and adoption of in vitro alternatives to animal testing in pharmaceutical drug development providing new opportunities to improve success rates coupled with a strong commitment to the 3Rs. In vitro research examines cells and biological molecules outside the living body, in a contained environment, typically a solution or a culture medium, often known as a Petri dish The term “in vitro” is derived from Latin meaning “in glass,” hence the popular term “test tube”. The BCOP assay uses excised animal tissue to replace the in vivo studies, and when used in combination with other in vitro tests, could fully replace the use of an animal model in the future 22.
In vitro systems are considered as alternative testing methods to reduce the use of animals in toxicity studies, refine toxicity evaluations (ie, to go beyond general growth or survival data), and replace in vivo studies—the socalled 3 Rs of alternative tests Cell or tissuebased approaches represent a more direct way to determine the mode of action of xenobiotics at the fundamental level. Making more use of invitro testing, the upcoming 21stcentury scientific fields known as 'omics' sciences and developing smart test strategies can clearly reduce the amount of essential animal. Animal test $32,000 in vitro test $11,000 Eye irritation/corrosion Draize rabbit eye test animal test $1,800 Bovine corneal opacity and permeability (BCOP) test in vitro test $1,400 Skin corrosion Draize rabbit skin test animal test $1,800 EpiDerm™ human skin model in vitro test $850 CORROSITEX® membrane barrier in vitro test $500 Skin sensitisation.
Animal test $32,000 in vitro test $11,000 Eye irritation/corrosion Draize rabbit eye test animal test $1,800 Bovine corneal opacity and permeability (BCOP) test in vitro test $1,400 Skin corrosion Draize rabbit skin test animal test $1,800 EpiDerm™ human skin model in vitro test $850 CORROSITEX® membrane barrier in vitro test $500 Skin sensitisation. Alternatives to animal testing include sophisticated tests using human cells and tissues (also known as in vitro methods), organsonchips, advanced computermodeling techniques (often referred to as in silico models), and studies with human volunteers These and other nonanimal methods are humane, and they aren’t hindered by species differences that make applying animal test results to humans difficult or impossible. A novel alternative approach that can identify chemicals which affect male reproductive health without the use of animal tests has been developed in a research project led by the Technical.
Other research methods such as in vitro testing (tests done on human cells or tissue in a petri dish) offer opportunities to reduce or replace animal testing 15 T Technological advancements in 3D printing allow the possibility for tissue bioprinting a French company is working to bioprint a liver that can test the toxicity of a drug 16 A. A novel alternative approach that can identify chemicals which affect male reproductive health without the use of animal tests has been developed in a research project led by the Technical. The EpiOcular EIT is an in vitro assessment assay that has the ability to differentiate articles that are ocular irritants from articles that are not ocular irritants The EIT is accepted as OECD TG 492 The Bovine Corneal Opacity and Permeability test method (BCOP) is an in vitro test method that can be used to classify substances as ‘ocular corrosives and severe irritants’.
MB Research Labs works closely with other leaders in the field of in vitro toxicology and is dedicated to advancing techniques that reduce the use of live animals in Toxicological Research Below are several related sources that are helpful in developing and validating novel in vitro toxicology testing methods National Toxicology Program. Invitro testing removes a small bit of tissue from a living body and tests the tissue itself, leaving the living organism safe and sound The Latin term in vitro means “in glass” and refers to the tissues being tested in glass test tubes Invitro testing is one of the many alternative options to avoid animal testing. Many acute toxicity tests have been, or are being, effectively replaced by in vitro human, animal, and 3D tissue alternatives This shift is particularly evident in skin irritation, skin corrosion, and skin sensitization models, all of which are fully supported here.
We have a longstanding, strong partnership with the Institute for In Vitro Sciences (IIVS), a nonprofit research and testing consortium dedicated to advancing the science of in vitro (nonanimal) methods worldwide IIVS develops and implements programs where alternatives to animal testing are currently not accepted. In vitro testing is great, but testing human tissues can be even more effective We can see how many groups of cells, perhaps from different areas of the body, react to chemicals and other products Technology on this front is advancing rapidly. Relies on data from animal testing Computer models and simulations.
Side a living organism and used for testing Although animals are still required as a source for these in vitro systems, the animal would experience distress for a much shorter time, and perhaps less distress overall, than occurs with wholeanimal testing because it would be killed before any experimental manipulations were carried out. If vaccine potency/efficacy testing is conducted in laboratory animal models or in vitro, separate safety tests in the targeted enduse animal are typically required Under certain circumstances, US regulations (9 CFR 1134) provide for exemption from testing, such as when consistency of safety is demonstrated in a sufficient number of. In light of these statistics, it’s clear that animal testing is not disappearing any time soon However, because of new innovations in the field of nonanimal testing, alternatives to animal experiments are now available in many cases In addition, more than 1,000 companies worldwide have already been certified as “crueltyfree” InVitro International is proud to offer multiple testing kits and laboratory services companies can use to replace animal tests.
Animalderived serum, especially foetal bovine serum (FBS), is widely used in in vitro assays as a supplement in cell culture media to support cell growth, but its production has animal welfare concerns and its use impacts on the reproducibility of experiments due to inherent batchtobatch variation (van der Valk et al, 18) The complete. A number of components of in vitro assays are currently derived from animal sources These include Metabolic Enzymes The provision of sources of metabolism for in vitro assays are mostly obtained from rat livers (Hubbard et al, 1985) Rats are dosed with a substance such as Aroclor or phenobarbital to induce increased levels of liver metabolism before being killed and an S9 fraction of their liver collected, which is then added to assays. Animals used in research are mainly small mammals such as guinea pigs, mice, rats, and rabbits Birds and fish are also used for specific experiments Mice make up 68% of animals used for testing, while rats make up 13% of the total number of animals used for testing Both mice and rats are also the most commonly used for organ extraction.
An overview of nonanimal methods that have been proposed for regulatory safety or efficacy testing of chemicals or biological agents can be found in the Tracking System for Alternative Methods (TSAR) resource, provided by the European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) TSAR tracks progress of an alternative method from submission for validation through. At InVitro International, we’re committed to helping companies implement crueltyfree testing practices with Irritection ® and Corrositex ®, two in vitro testing methods that don’t rely on live animal models In addition, we can help manufacturers of all sizes improve their testing practices without increasing inhouse lab tests with our own stateoftheart testing services, performed using our patented methods. Observing the effects of drugs in the body, studying brain structure and function, studying brain diseases and other neurological disorders Cannot reveal all drug effects in the body;.
In vitro testing is going to protect animals and it is actually more effective because animal responses will differ from a human response The organsonchips use actual human cells, therefore the cell response is way more efficient than testing on animals. The BCOP assay uses excised animal tissue to replace the in vivo studies, and when used in combination with other in vitro tests, could fully replace the use of an animal model in the future 22. In vitro studies, which are performed on cultured bacterial or mammalian cells, can be used as an initial screen to avoid the unnecessary use of animals in determining which candidates should move forward for further safety testing They are also used to replace many of the acute studies which have historically been performed in animals.
Determining drug safety and efficacy requires testing in animals. Unique among journals in its focus on in vitro biology in animals Offers studies of cellular, molecular, and developmental biology that employ or are relevant to organs, tissue, tumors, and cells in vitro Peerreviewed coverage of stateoftheart research since 1965 A journal of the Society for In Vitro Biology. In vitro tests also take around two weeks in total to analyse, less than the four weeks for an animal trial The test is also said to be cheaper than animal testing methods Human skin sensitisation tests are important to determine the safety of cosmetics, but difficult to replicate and measure due to the need for an immune response.
During the last decade, many in vitro tests emerged These are based on animal cells, human tumour cell lines, primary cells, immortalized cell lines, embryonic stem cells, or induced pluripotent stem cells They differ in their readouts and range from simple viability assays to complex functional endpoints such as neural crest cell migration. Invitro toxicology testing is commonly employed by the pharmaceutical, cosmetic, chemical, food, medical device and diagnostics industries to test the safety (toxicology/toxicity) and efficacy of chemicals, biochemicals, materials, preparations and vaccines It offers an effective and everimproving alternative to animal testing. Yes and no To a large extent the animaltesting that was used in pharmaceutical development has been replaced by insilico and invitro methods These are used to screen out products before they reach the animaltesting stage.
ANIMAL TESTS VS IN VITRO* ALTERNATIVES * “In Vitro” refers to a test that uses cells or tissue contained in testtubes or other laboratory equipment TYPE OF TEST COST Genetic toxicity Chromosome aberration animal test $30,000 in vitro test $,000 Sister chromatid exchange animal test $22,000 in vitro test $8,000. EU, invitro testing replaces animal testing completely In other areas, it can be used to reduce the number of animals and tests required, or refine procedures to limit animal impacts • Under REACH, skin corrosion tests are key parameters in the assessment of a chemical for registration with the European Agency (ECHA). A number of components of in vitro assays are currently derived from animal sources These include Metabolic Enzymes The provision of sources of metabolism for in vitro assays are mostly obtained from rat livers (Hubbard et al, 1985) Rats are dosed with a substance such as Aroclor or phenobarbital to induce increased levels of liver metabolism before being killed and an S9 fraction of their liver collected, which is then added to assays.
In vitro testing Cells or tissue samples taken from animals or humans and prepared for laboratory study Drug research, testing new chemicals and products on human skin, toxicology testing In vitro techniques focus on the cellular level and therefore cannot replace wholebody testing;. In Vitro Testing Using human cells instead of live animals is not only a crueltyfree way to test consumer products, medications, and more, but also a more reliable indicator of how that product will interact with human beings As mentioned above, animals don’t have our exact biological makeup. In vitro In vitro testing occurs in a laboratory and usually involves studying microorganisms or human or animal cells in culture This methodology allows scientists to evaluate various biological.
Today—because experiments on animals are cruel, expensive, and generally inapplicable to humans—the world’s most forwardthinking scientists have moved on to develop and use methods for studying diseases and testing products that replace animals and are actually relevant to human health These alternatives to animal testing include sophisticated tests using human cells and tissues (also known as in vitro methods), advanced computermodeling techniques (often referred to as in silico. IIVS offers news & resources on the latest in the in vitro testing industry IIVS, RIFM and Shiseido Partner to Transfer Nonanimal Photosafety Tests The Institute for In Vitro Sciences (IIVS) has entered into an agreement with Shiseido and the Research Institute for Fragrance Materials (RIFM) to support the technology. At this time, the primary use of invitro tests is the detection of specific toxic properties of drugs and chemicals, eg, mutagenesis and mechanisms of toxicity The theoretical basis for this stems from the commonality of the basic structure and behavior of genetic material whereby invitro tests for genetic toxicology can replace animals tests.
A combination of in vitro test methods and already available human and animal data may be used to determine the potential for skin sensitization instead of using the traditional guinea pig and mouse tests. Introduction Scientists often study the effects of drugs and chemicals on animals before they deem them safe for humans When possible, they try to perform these toxicology tests using biochemical or cellbased ( in vitro) systems instead of with animals such as mice. Live animals and embryos are used to study effects of some compounds on embryo development In vitro embryonic stem cell culture test helps to reduce the number of live embryo used and the compounds which are toxic toward developing embryo (Gipson and Sugrue, 1994, De Silva et al, 1996) Also, sharing or providing the discovered data (like characteristics of excipients for the test drug) avoids the necessity of animal studies.
During the last decade, many in vitro tests emerged These are based on animal cells, human tumour cell lines, primary cells, immortalized cell lines, embryonic stem cells, or induced pluripotent stem cells They differ in their readouts and range from simple viability assays to complex functional endpoints such as neural crest cell migration. In vitro embryonic stem cell culture test helps to reduce the number of live embryo used and the compounds which are toxic toward developing embryo (Gipson and Sugrue, 1994, De Silva et al, 1996) Also, sharing or providing the discovered data (like characteristics of excipients for the test drug) avoids the necessity of animal studies 22.

In Vitro The Remarkable Rise Of Animal Alternatives Understanding Animal Research Understanding Animal Research
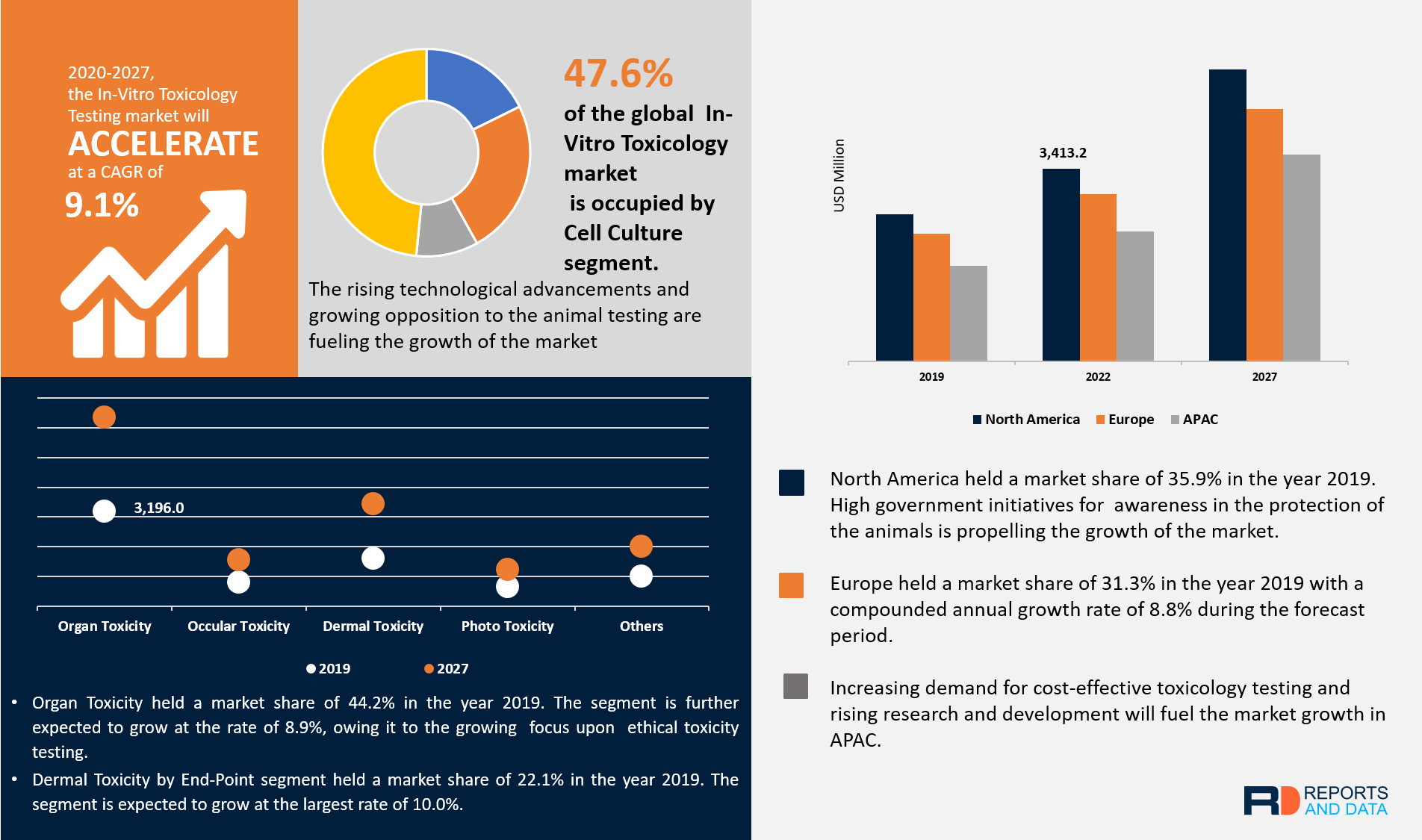
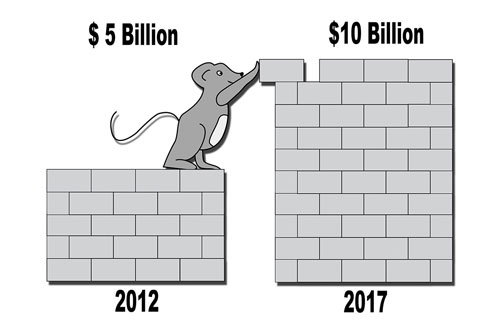
In Vitro Toxicology Toxicity Testing Market Size Share 17 27
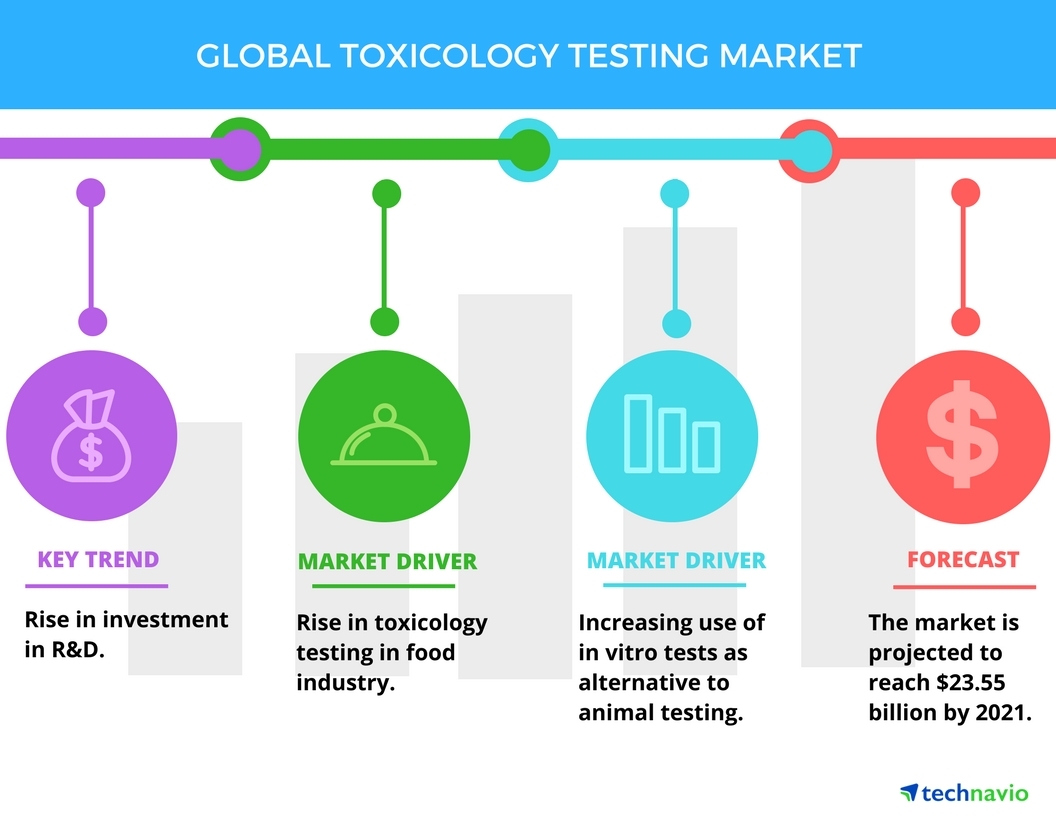
Top 3 Drivers In Toxicology Testing Market Technavio Business Wire
In Vitro Animal Testing のギャラリー

Xcellr8 Signs Licencing Deal With Senzagen To Extend Gard Animal Free Testing
Q Tbn And9gctmscmwwsxadnmpe3rcaapywshoc3fdbtvg6javfym H1wcygfh Usqp Cau
Naturewatch Foundation Global Market For In Vitro Toxicology Testing Is More Reliable Than Animal Testing Predicted To Grow To 8 8 Bn By 23 T Co Nv8i0uvyk7 Animaltesting T Co Y9pbdxppb7

Alternatives To Animal Testing Cruelty Free International

Henkel Helps Fight Against Animal Testing With Unlimited Access To In Vitro Bioartificial Human Skin Models Global Cosmetics News

In Vitro Non Animal Testing Using The Reconstructed Human Epidermis Model Cyprotex

Animal Welfare Experimentica Ltd
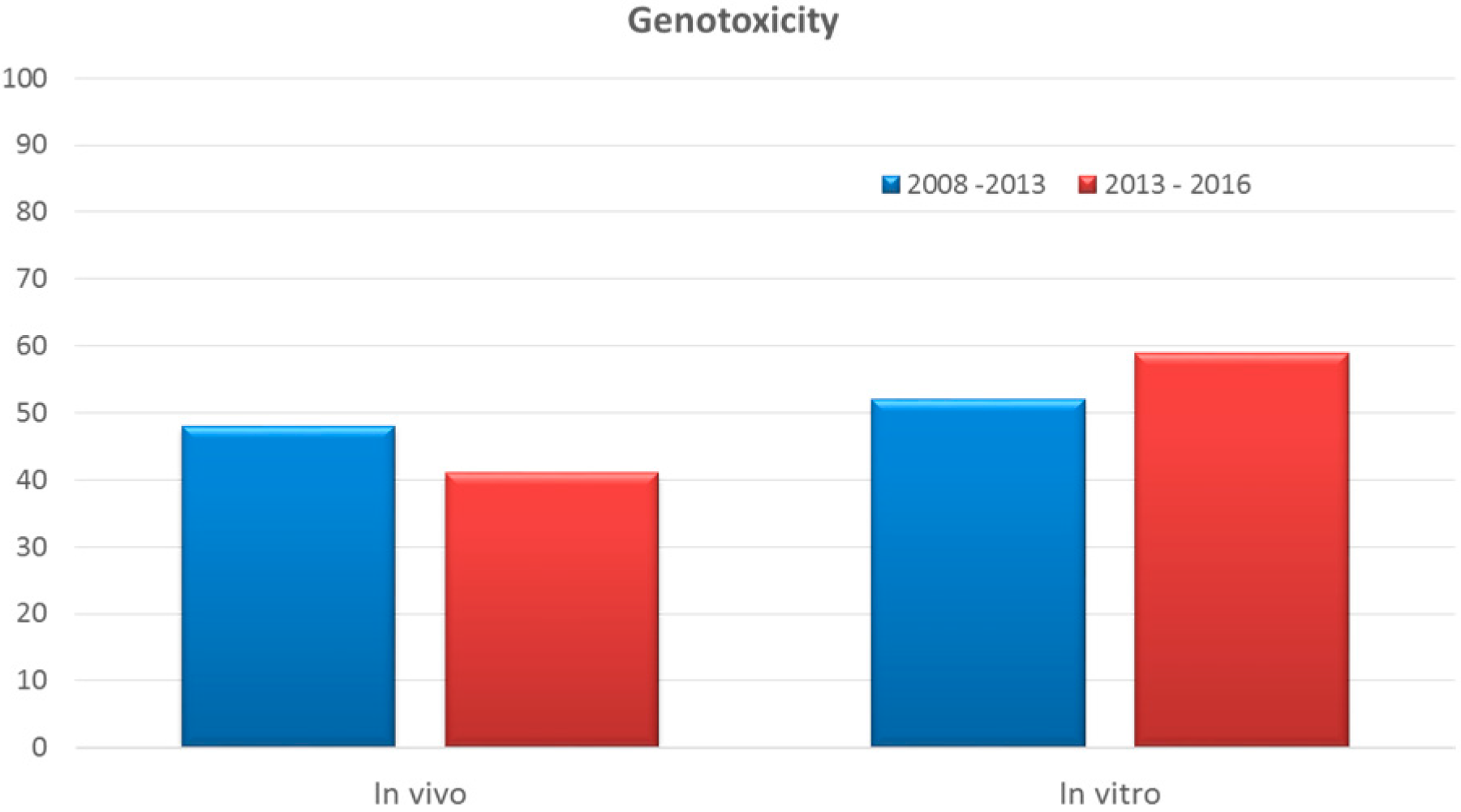
Cosmetics Free Full Text Alternative Methods To Animal Testing For The Safety Evaluation Of Cosmetic Ingredients An Overview

Alternatives To Animal Testing Wikipedia
In Vitro Methods And More Animal Testing Alternatives Peta

Computer Techniques Aim To Save Animals From Experiments News Communications Of The Acm

Providing Safe Products Sustainability Mitsui Chemicals Inc
Non Animal Testing In Vitro Toxicology Alternative Testing
Vito Leading In Developing Animal Free Testing Methods Vito

In Vitro Toxicity Testing Market Global Forecast To 25 Marketsandmarkets

Pdf Alternatives To Animal Testing In The Safety Evaluation Of Products
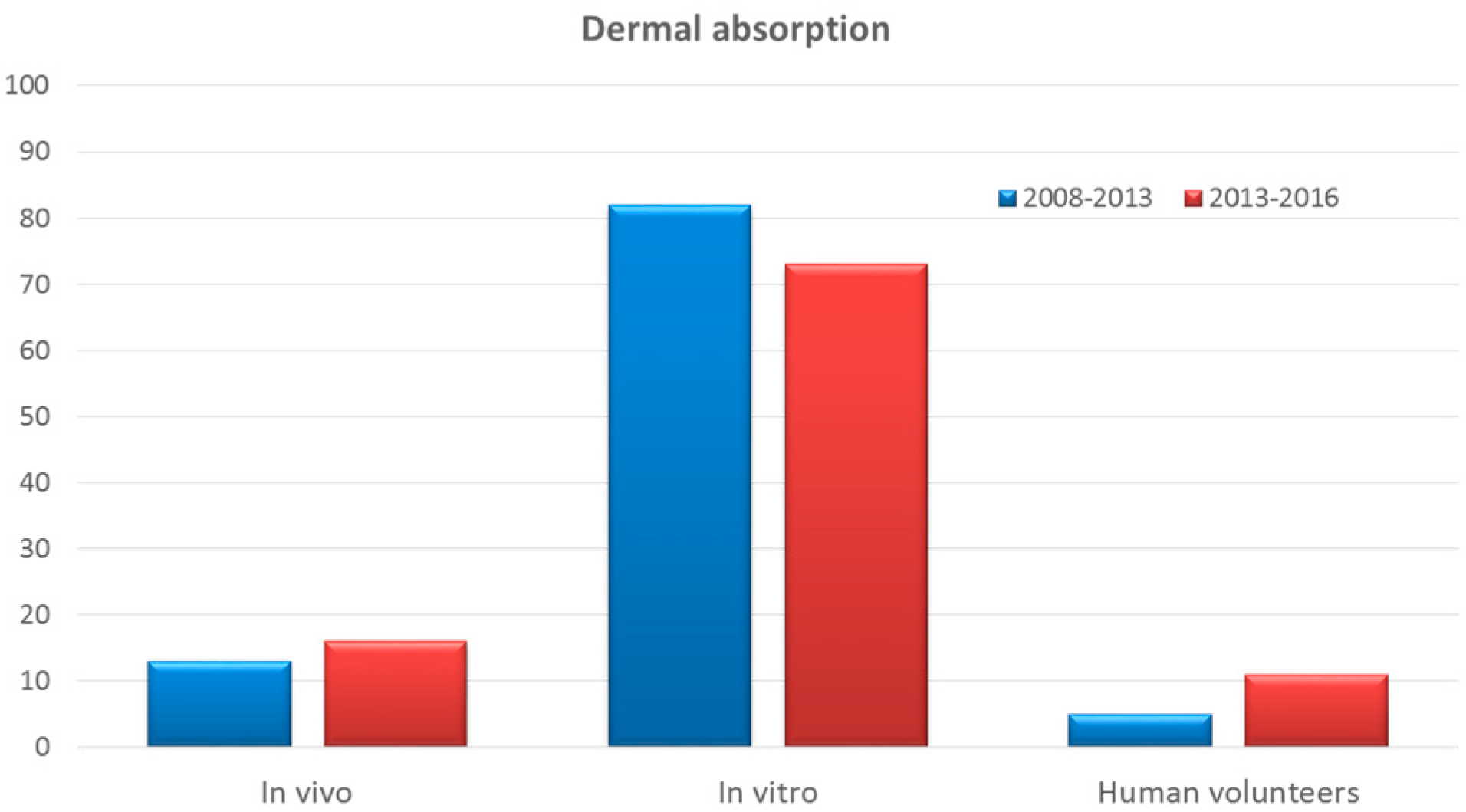
Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd
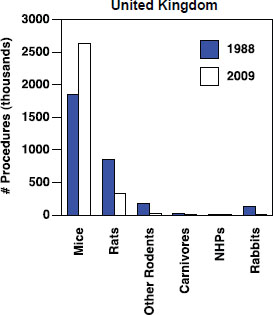
4 Animals In Neuroscience Research International Animal Research Regulations Impact On Neuroscience Research Workshop Summary The National Academies Press

Animal Testing Alternatives Must Be Eu Priority Says Cruelty Free Europe Can Cosmetics Lead

Laboratories Abich It Biological And Chemical Analysis

Better Animal Testing Alternatives Are Coming To U S Mddionline Com

Pdf Eurl Ecvam Strategy To Avoid And Reduce Animal Use In Genotoxicity Testing Semantic Scholar

Safety Methods For Eye And Skin Irritation Tests Invitrointl
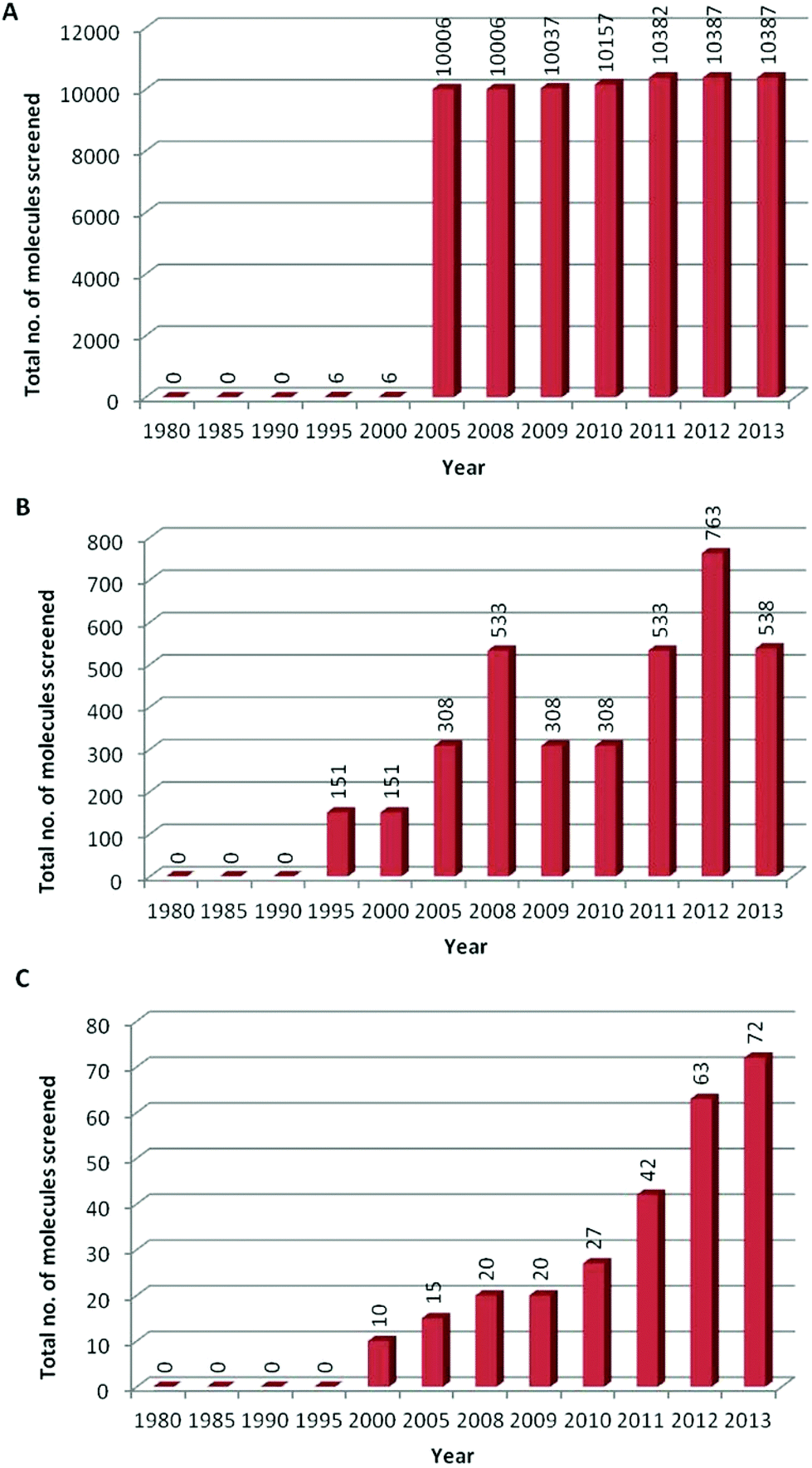
Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd
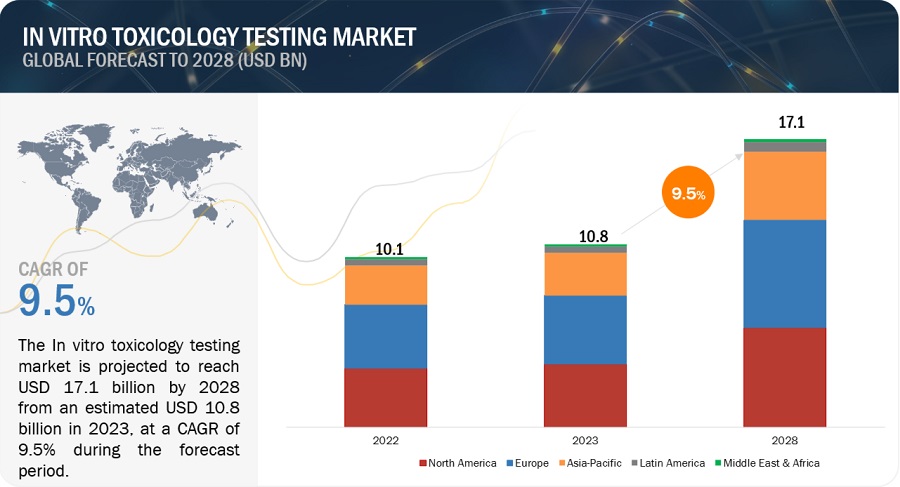
In Vitro Toxicity Testing Market Global Forecast To 25 Marketsandmarkets
Www Dsm Com Content Dam Dsm Corporate En Us Documents Position Paper Animal Studies Pdf

Animal Tests Ban For Cosmetics Debate On A Ep S Resolution 16 November Palace Of Nations Geneva Oipa

Reach A Better Solution In Vitro Skin Sensitization Assays As Animal Testing Alternatives
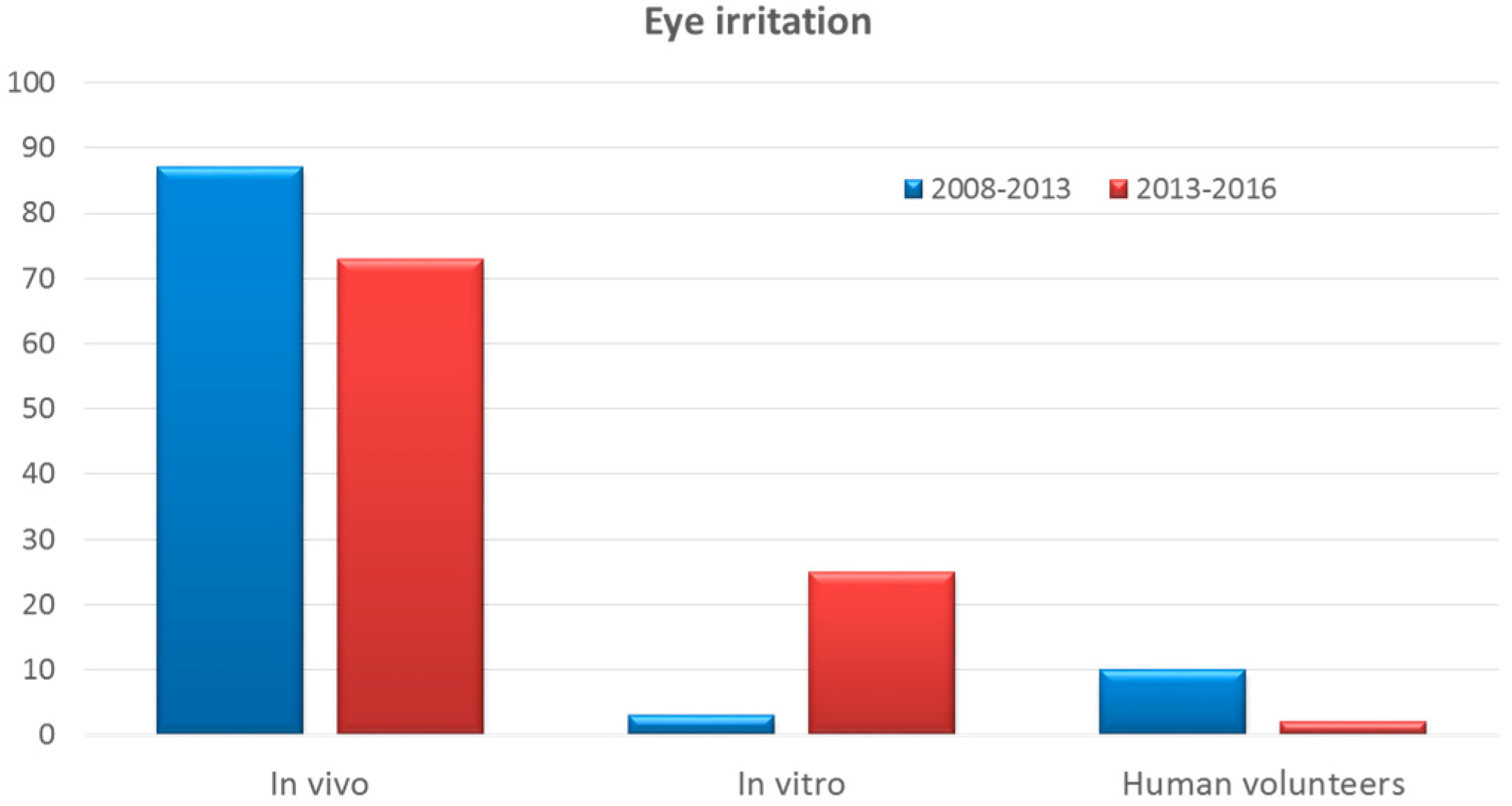
Cosmetics Free Full Text Alternative Methods To Animal Testing For The Safety Evaluation Of Cosmetic Ingredients An Overview

Epa Recommends Moving Away From Animal Testing While Big Data Shows Testing Is Often Unnecessary Invitrointl

In Vitro Testing

Eurl Ecvam Issues Recommendation On Three Alternative To Animal Testing Methods For Carcinogenicity Eu Science Hub

Reach Taking Up Alternatives To Animal Testing Kosmetica World

Us Environmental Agency To End Animal Testing By 35 Laboratory News

Announcements Altex Alternatives To Animal Experimentation
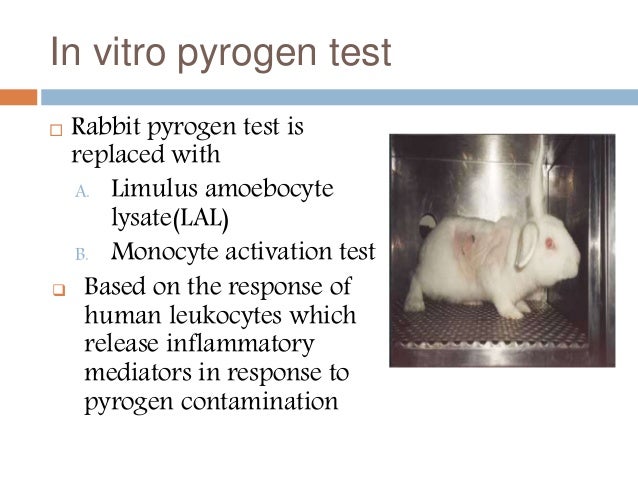
Alternatives To Animal Experiments

In Vitro Citizens For Alternative To Animal Research

Alternatives To Animal Screening Methods P Screening Mohammadhusain
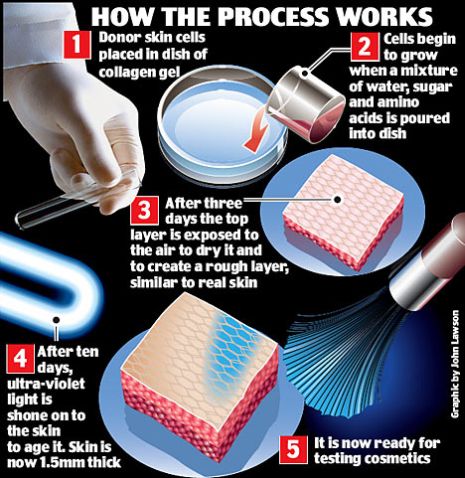
Available Alternatives Alternatives To Animal Testing
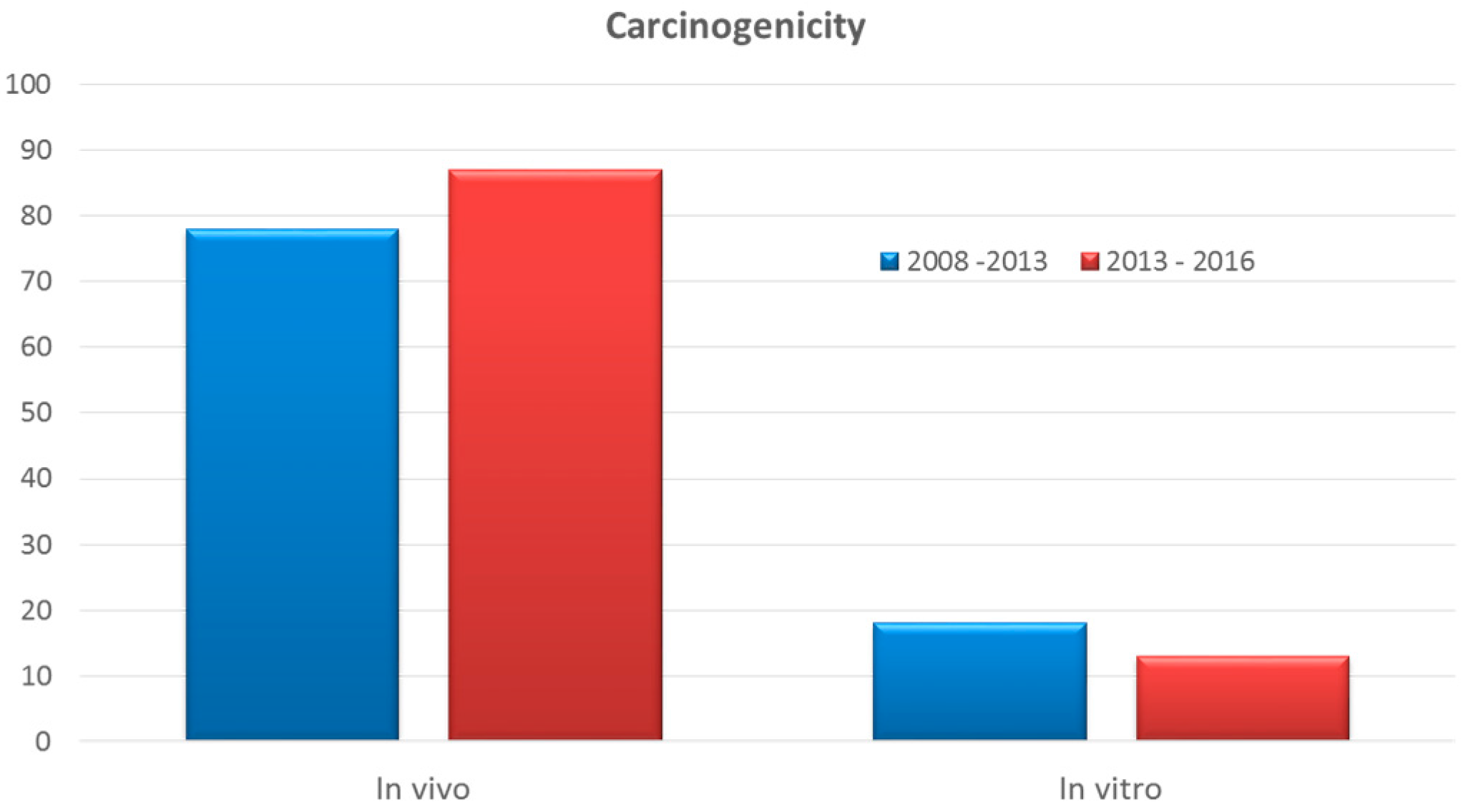
Cosmetics Free Full Text Alternative Methods To Animal Testing For The Safety Evaluation Of Cosmetic Ingredients An Overview Html

Top Five Reasons To Stop Animal Testing Peta
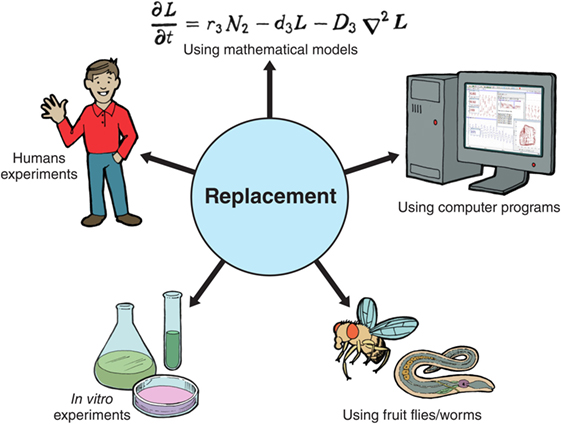
The 3rs What Are Medical Scientists Doing About Animal Testing Frontiers For Young Minds
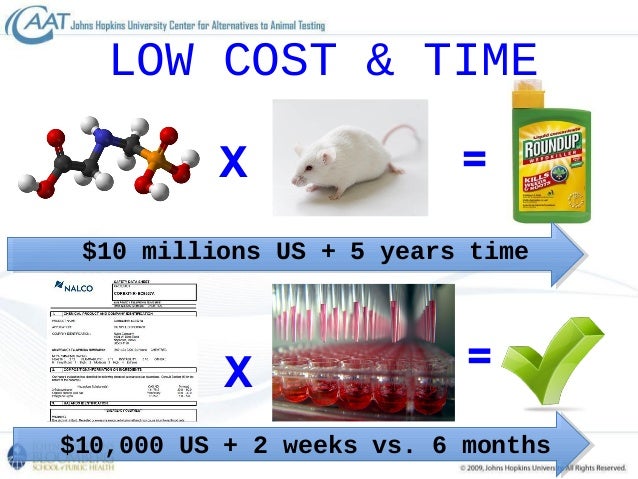
Animal Testing Science Or Tradition

Uk Government Bans Animal Testing On Household Products Cyprotex

In Vitro Toxicology Toxicity Testing Market Global Opportunity Analysis And Industry Forecast 19 25 Meticulous Market Research Pvt Ltd

Ready To Use Pyromat In Vitro System

In Vitro Testing Archives Invitrointl

Animal Welfare And Non Animal Testing For Regulatory Purposes Croner I

In Vitro Tissue Based Models As A Replacement For Animal Models In Testing Of Drugs At The Preclinical Stages Intechopen

Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd

Selected In Vitro And In Vivo Testing Methods For Short Term Download Table

In Vitro Genotoxicity Testing Can The Performance Be Enhanced Sciencedirect

How Omics Technologies Can Contribute To The 3r Principles By Introducing New Strategies In Animal Testing Trends In Biotechnology
Cruelty Free In Vitro Breakthrough Could End Animal Testing For Carcinogenic Substances Livekindly

Mat A Validated In Vitro Assay For Detection Of All Pyrogens

Preclinical Animal Testing Requirements And Considerations Sciencedirect
The Alternatives To Animal Testing Animals Are Not Ours To Experiment On Peta Uk

What Is Animal Testing Facts Pros Cons Alternatives
Alternatives To Animal Testing Drive Market

Alternates To Animal Testing Dabur Research Foundation

Alternatives To Animal Testing
1
Cosmetics Free Full Text Alternative Methods To Animal Testing For The Safety Evaluation Of Cosmetic Ingredients An Overview Html

Our Commitment No Animal Testing For Our Products

Pharmaceutical Toxicology Designing Studies To Reduce Animal Use While Maximizing Human Translation Sciencedirect

In Vitro Tests Replacing Test Animals In The Quality Control Of Download Table

Cellsine Looking To Reduce Your Reliance On Animal Testing Come And Visit Cellsine At The Ic 3rs Symposium On May 8th In Brussels And See How We Can Improve Your In Vitro

Alternatives To Animal Testing Wikipedia
Animal Testing And Alternatives

Echa Animal Testing Alternatives Report For Reach Regulation

Animal Testing Pros Cons Procon Org

Animal Testing Labs In India Cognibrainmanuscript

In Vitro Toxicology Toxicity Testing Market To Grow At A Cagr Of 9 From 19 To Reach 14 4 Billion By 25 Meticulous Research Marketersmedia Press Release Distribution Services News Release Distribution Services

Reducing Animal Testing Using Stem Cells

Julie Hurst Hurstj3 Profile Pinterest

Shiseido S In Vitro Test More Accurate Than Animal Testing

Can Animal Research Be Applied To Humans Speaking Of Research

Animal Testing In Eu Declining Cosmetics Must Share In Vitro Methods
Q Tbn And9gcqpeebnbvwurw3t8efwncylvuhh S0tmsj9rfznwxsoagxomkiw Usqp Cau
Alternatives To Animal Testing The Future Of R D By Anubha Mehra Medium
Hsi India Teams Up With Reagene Biosciences To Advance Non Animal Testing Research In India Humane Society International
Q Tbn And9gctyvrlpdn74l3iruwq5crvuu3rdjggog3u2ri49fyoikmikugyf Usqp Cau

Pdf Animal Testing And Its Alternatives The Most Important Omics Is Economics

Animal Research Statistics For Germany In 13 Speaking Of Research

Research Animal Fundamentals Sources Faunalytics

Singapore Scientists Create In Vitro Human Skin As Alternative To Animal Testing Global Cosmetics News

Alternatives Stop Animal Testing
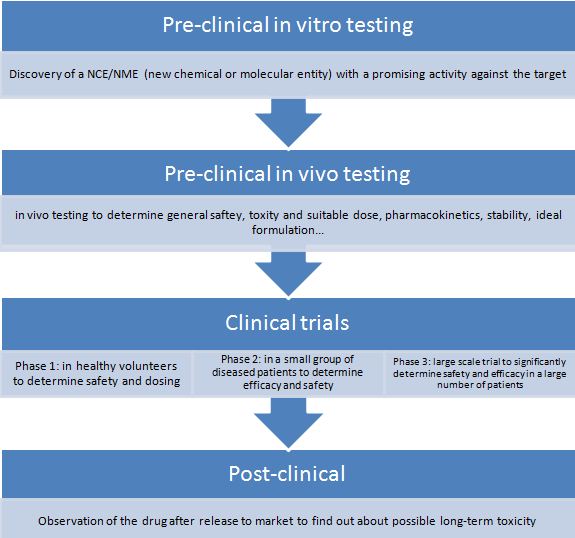
Animal Testing The Bridge Between English2biotech

In Vitro Non Animal Testing Using The Reconstructed Human Epidermis Model Cyprotex

The Use Of Various Approaches To Phase Out Animal Testing Download Scientific Diagram

Echa Newsletter Home

Saving The Animals New Ways To Test Products The New York Times

Figure 3 From Animal Testing And Its Alternatives The Most Important Omics Is Economics Semantic Scholar

Alternatives To Animal Testing

Feed Quilting Testing Measuring In Vivo In Vitro Digestibility Global Aquaculture Advocate
In Vitro Methods And More Animal Testing Alternatives Peta

Zhejiang Releases 3 New In Vitro Test Standards For Cosmetics Chemlinked
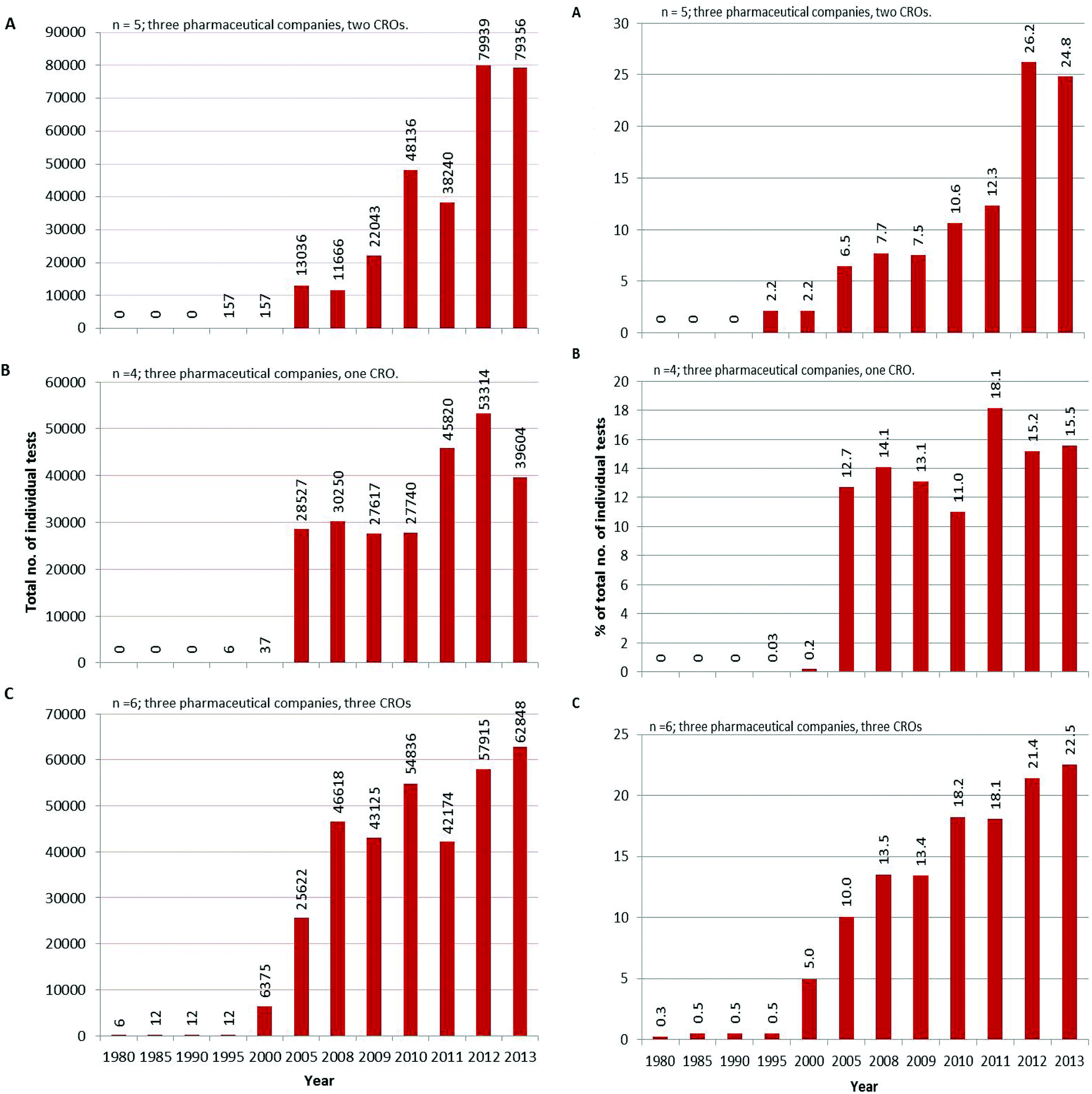
Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd

Alternates To Animal Testing Dabur Research Foundation
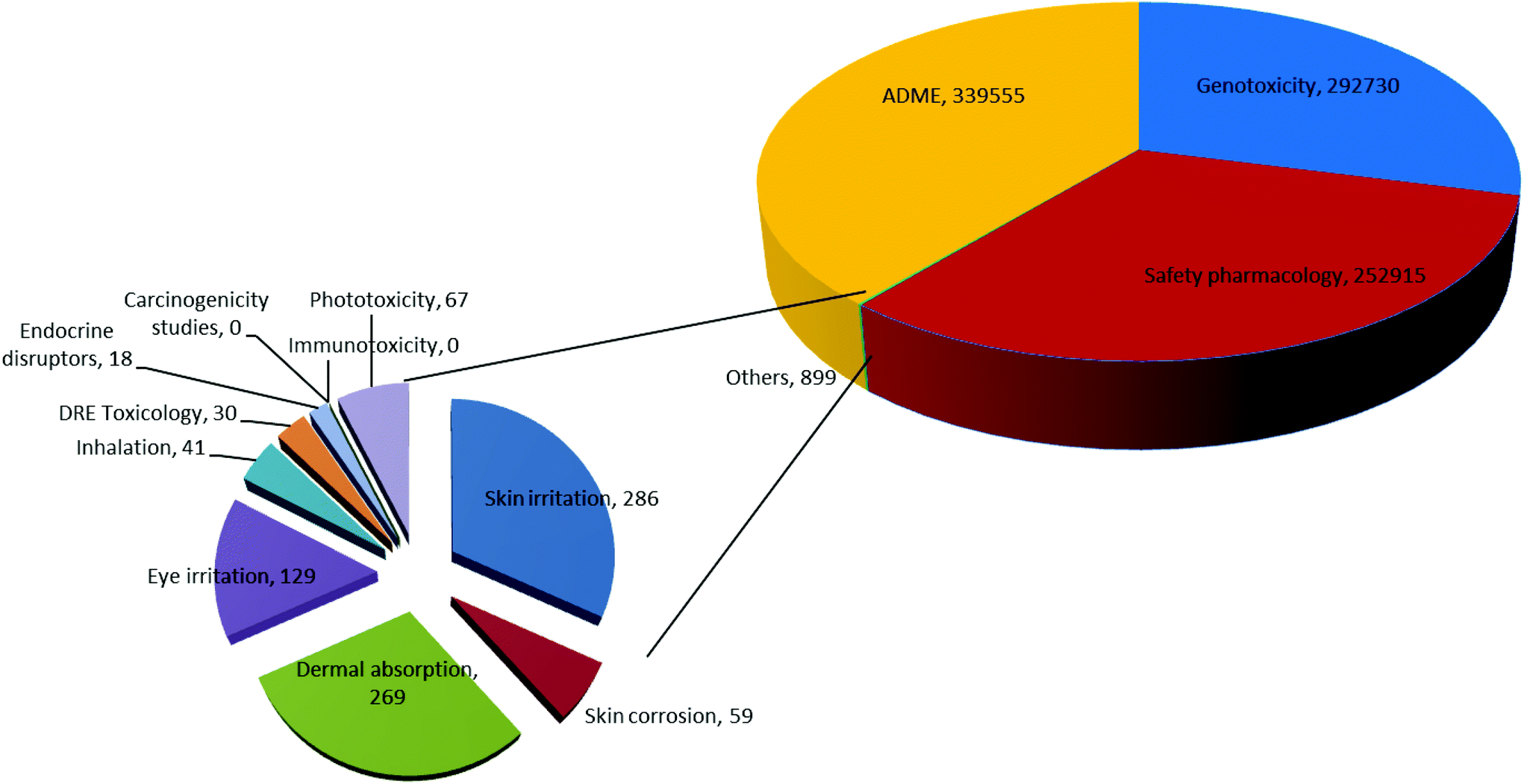
Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd
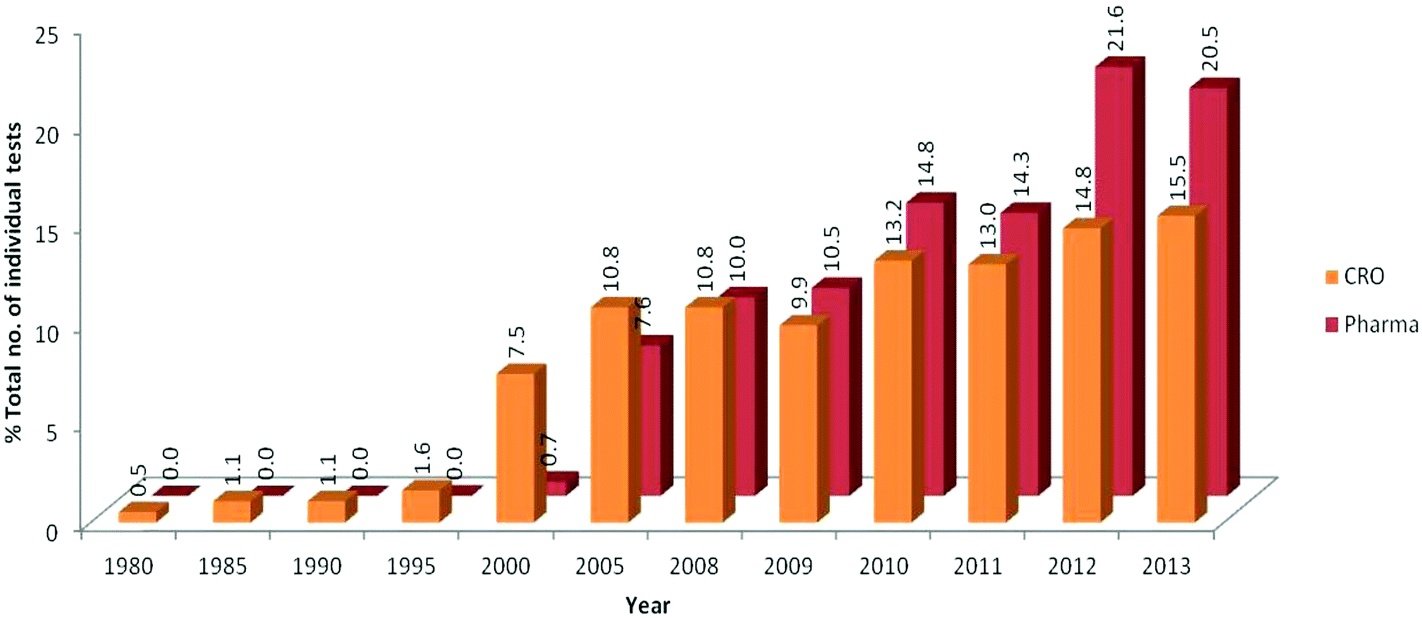
Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd



