In Vitro Toxicity Testing
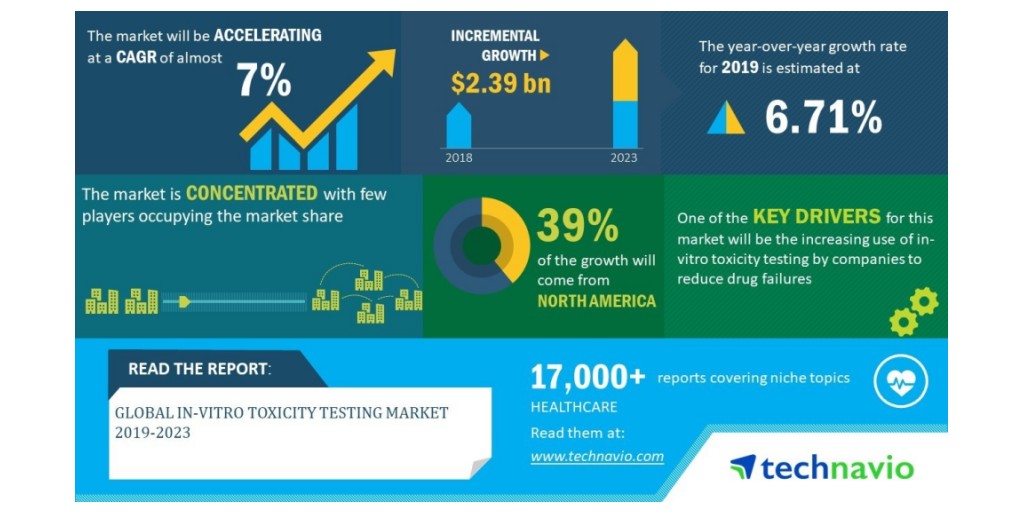
The global in vitro toxicity (predictive toxicity) testing market is valued at more than $13 billion in 10 With heightened awareness of animal welfare in laboratory research testing, the in vitro toxicity testing market has an anticipated value of $27 billion in 15, a compound annual growth rate (CAGR) of 15% between 10 and 15.

In vitro toxicity testing. An array of in vitro testing methods can be used to verify that cosmetic products and ingredients are free from components that are cytotoxic, mutagenic, or capable of causing skin and eye irritation (temporary damage) or corrosion (permanent damage) Efficacy and ingredient claims. In Vitro Toxicity Tests In vitro testing is a crucial part of an innovative testing strategy, using tiered approaches to identify ENM, which should undergo all testing steps including animal tests An approved testing scheme would allow new regulations and test guidelines to reduce testing efforts. Currently used in vitro assays do not adequately predict in vivo observed effects, predominantly due to an inadequate preservation of the organs’ microenvironment in the models applied The kidney is highly complex, composed of a filter unit and a tubular segment, together containing over different cell types.
Invitro toxicology testing is commonly employed by the pharmaceutical, cosmetic, chemical, food, medical device and diagnostics industries to test the safety (toxicology/toxicity) and efficacy of chemicals, biochemicals, materials, preparations and vaccines It offers an effective and everimproving alternative to animal testing. Stemina Biomarker Discovery has pioneered building human cellular models for toxicity screening of drug candidates, chemical compounds, and cosmetic and tobacco ingredients Our in vitro toxicity assays offer an alternative to animal tests that are highly predictive of human response using human cellbased endpoints and very little test compound These assays can provide data in only a few weeks at a fraction of the cost of animal testing. In vitro toxicity testing is the scientific analysis of the effects of toxic chemical substances on cultured bacteria or mammalian cells In vitro testing methods are employed primarily to identify potentially hazardous chemicals and/or to confirm the lack of certain toxic properties in the early stages of the development of potentially useful new substances such as therapeutic drugs, agricultural chemicals and food additives In vitro assays for xenobiotic toxicity are recently carefully consid.
There is an urgent need to develop in vitro assays that correlate with in vivo toxicity tests in the study of venoms and in the assessment of the neutralizing ability of antivenoms, along with the 3Rs paradigm This goal must be strengthened by research funding agencies and agendas, regulatory agencies and diverse stakeholders related to. In vitro toxicity tests have been wellestablished to support the needs of highthroughput screening in new drug development Invitrocue offers single dose and repeated dose toxicity tests that conform to regulatory guidelines for your R&D needs Dose finding (LD50). Acute inhalation toxicity testing for regulatory purposes is currently performed only in rats and/or mice according to OECD TG403, TG436, and TG433 test guidelines Such tests are biased by the differences in the respiratory tract architecture and function across species, making it difficult to draw conclusions on the potential hazard of inhaled compounds in humans.
Dermatological invitro safety testing and clinical trials, taken together, lead to wellsubstantiated product claims that are key to ensuring your product’s success Our Capabilities CPT has stateoftheart analytical, microbiological, clinical, photobiology, and invitro toxicology testing laboratories , all of which are at your disposal. Invitro toxicity testing involves the use of cultured cells or computer models to identify toxic or hazardous chemicals of new substances These tests can also be used to confirm the absence of toxic chemicals from the drug or substance of choice Invitro toxicity testing is usually prevalent in the early stages of clinical trials. Detailed report on In Vitro Toxicology Market scrutinized in new research 21 Get a sample brochure @ http//tinyurlcom/hgx36dx Scientific analysis of the effects of toxic chemical substances on mammalian cells is called in vitro toxicology testing.
Market Study Report adds new report on Global InVitro Toxicology and Toxicity Testing Market analysis 25 The report focuses on global major leading industry players with information such as company profiles, end users/applications, product and specification. The Invitro Toxicity Testing market was valued at XX Million US$ in 18 and is projected to reach XX Million US$ by 24, at a CAGR of XX% during the forecast period In this study, 18 has been considered as the base year and 19 to 24 as the forecast period to estimate the market size for Invitro Toxicity Testing. Medical Device Testing In vitro& in vivobiological compatibility/safety evaluations are conducted on biomaterials, medical devices, and related products to identify potential risks for the use of a device in humans Biocompatibility testing ranges from the screening of new materials to product release testing, audit testing, and premarket safety.
The global invitro toxicology testing market is expected to reach USD 1042 Billion by 25, from USD 63 Billion in 17 growing at a CAGR of 65% during the forecast period of 18 to 25 The upcoming market report contains data for historic years 17, the base year of calculation is 17 and the forecast period is 18 to 25. The global invitro toxicology testing market is expected to reach USD 1042 Billion by 25, from USD 63 Billion in 17 growing at a CAGR of 65% during the forecast period of 18 to 25 The upcoming market report contains data for historic years 17, the base year of calculation is 17 and the forecast period is 18 to 25. In vitro methods are mainly used for skin/eye irritation test, skin sensitisation test and genotoxicity tests They cannot replace acute toxicity, repeated dose toxicity, development and reproductive toxicity, and carcinogenicity yet Factors to Consider When Using In Vitro Methods.
Certain chemicals, if present in cosmetics products, even in very small doses, can cause temporary or permanent damage to human skin, eyes, and DNA An array of in vitro testing methods can be used to verify that cosmetic products and ingredients are free from components that are cytotoxic, mutagenic, or capable of causing skin and eye irritation (temporary damage) or corrosion (permanent damage). The global in vitro toxicology testing market is estimated to be valued at USD 918 billion in and is projected to grow at a CAGR of 103% during the forecast period of to 25. In vitro toxicity tests have been wellestablished to support the needs of highthroughput screening in new drug development Invitrocue offers single dose and repeated dose toxicity tests that conform to regulatory guidelines for your R&D needs Dose finding (LD50).
In vitro toxicity tests have been wellestablished to support the needs of highthroughput screening in new drug development Invitrocue offers single dose and repeated dose toxicity tests that conform to regulatory guidelines for your R&D needs Dose finding (LD50). Before submitting an IND application, the concerned drug must go through a comprehensive series of invitro and invivo toxicity testing to ensure maximum safety in clinical trials Considering the ethical issues and the cost of invivo animal tests, the pharmaceutical industry now relies more on invitro methods for toxicity testing in the drug development phase. In vitro toxicology is one of the most rapidly expanding areas of biological research today It is generally conceded that this is a result of pressure from the public for safer products and environmental conditions and, in these recessionary times, pressure from company accountants, who often perceive in vitro experimentation as a cheaper option than lawsuits, fines, and expensive remediation.
Invitro Toxicology Testing Market – Growth, Trends And Forecasts (25) The Invitro Toxicology Testing Market report is a compilation of firsthand information, qualitative and quantitative. Invitro toxicology testing is commonly employed by the pharmaceutical, cosmetic, chemical, food, medical device and diagnostics industries to test the safety (toxicology/toxicity) and efficacy of chemicals, biochemicals, materials, preparations and vaccines It offers an effective and everimproving alternative to animal testing. In vitro, ex vivo, and in silico testing methods are some of the alternatives to animal testing and are used to determine the toxicity of a substance Safety assessment and efficacy testing is a mandatory procedure for industries such as chemicals, pesticides, cosmetics, consumer products, drugs, vaccines, and medical devices.
Suggested Citation"2 Animal and In Vitro Toxicity Testing"National Research Council 06 Toxicity Testing for Assessment of Environmental Agents Interim ReportWashington, DC The National Academies Press doi /. Market Study Report adds new report on Global InVitro Toxicology and Toxicity Testing Market analysis 25 The report focuses on global major leading industry players with information such as company profiles, end users/applications, product and specification. The In Vitro Toxicity Testing of Tobacco Smoke Task Force was established in 02 to ensure that CORESTA provides leadership on assessing toxicity evaluation The first phase was stated as follows To prepare a report covering the rationale and strategy for conducting in vitro toxicity testing of cigarette smoke.
In vitro toxicity tests have been wellestablished to support the needs of highthroughput screening in new drug development Invitrocue offers single dose and repeated dose toxicity tests that conform to regulatory guidelines for your R&D needs Dose finding (LD50). Kidneybased in vitro models for druginduced toxicity testing Druginduced kidney injury Exposure to various drugs or drug candidates for therapeutic or diagnostic purposes (Fig 3) Models for nephrotoxicity screening Until recently, researchers were left with only two options in vitro. In vitro systems are considered as alternative testing methods to reduce the use of animals in toxicity studies, refine toxicity evaluations (ie, to go beyond general growth or survival data), and replace in vivo studies—the socalled 3 Rs of alternative tests Cell or tissuebased approaches represent a more direct way to determine the mode of action of xenobiotics at the fundamental level.
Offering acute toxicity, pharmacological, analytical chemistry, product safety testing, and alternative in vitro methods, featuring local lymph node assay USDA licensed with EPA and FDA inspections, in GLP facilities at Spinnerstown, Pennsylvania. Comprehensive In Vitro Toxicity Testing of a Panel of Representative Oxide Nanomaterials First Steps towards an Intelligent Testing Strategy Nanomaterials (NMs) display many unique and useful physicochemical properties However, reliable approaches are needed for risk assessment of NMs. In Vitro Toxicity Testing In vitro toxicology screening approaches are key tools to decrease the attrition of novel drug candidates as they progress through the discovery and development process.
The InVitro Toxicology and Toxicity Testing Market Report provides data on InVitro Toxicology and Toxicity Testing patterns and improvements, and target business sectors and materials, limits. Invitro tests for the longterm safety evaluation of drugs offer certain advantages Specific properties of drugs can be identified including mutagenic and carcinogenic effects The mechanisms leading to toxicity can be assessed Tissue from several species, including man, can be examined These te. The InVitro Toxicology and Toxicity Testing Market Report provides data on InVitro Toxicology and Toxicity Testing patterns and improvements, and target business sectors and materials, limits.
Introduction In vitro toxicology is one of the most rapidly expanding areas of biological research today It is generally conceded that this is a result of pressure from the public for safer products and environmental conditions and, in these recessionary times, pressure from company accountants, who often perceive in vitro experimentation as a cheaper option than lawsuits, fines, and expensive remediation. An in vitrotest method in which a test substance is applied to a semipermeable membrane measured to assess the test substance’s potential to cause eye irritation Can be used to determine the irritation potential of cosmetics, creams, and a wide variety of consumer products. The global invitro toxicology testing market is expected to reach USD 1042 Billion by 25, from USD 63 Billion in 17 growing at a CAGR of 65% during the forecast period of 18 to 25 The upcoming market report contains data for historic years 17, the base year of calculation is 17 and the forecast period is 18 to 25.
Leader in In Vitro Toxicology Field, Rigorous Scientific & Compliance Programs, Promotes Use & Regulatory Acceptance of Alternative Testing Methods Worldwide, Search About IIVS Team IIVS offers news & resources on the latest in the in vitro testing industry IIVS, RIFM and Shiseido Partner to Transfer Nonanimal Photosafety Tests. The Eurofins Discovery portfolio also includes a variety of other in vitro toxicology services, including testing for nephrotoxicity, neurotoxicity, and other target organ toxicities in a wide range of cell lines, metabolismmediated and mechanismbased toxicities, reactive metabolite detection using trapping agents, mitochondrial membrane potential and mitotoxicity, and a range of testing services to help investigate toxicities related to poor ADME PK properties, DrugDrug Interactions, and. The Invitro Toxicology Testing Market report is a compilation of firsthand information, qualitative and quantitative assessment by industry analysts, inputs from industry experts and industry.
Market Study Report adds new report on Global InVitro Toxicology and Toxicity Testing Market analysis 25 The report focuses on global major leading industry players with information such as company profiles, end users/applications, product and specification. Market Study Report adds new report on Global InVitro Toxicology and Toxicity Testing Market analysis 25 The report focuses on global major leading industry players with information such as company profiles, end users/applications, product and specification. Jan 15, 21 (Market Insight Reports) The Global Invitro Toxicology Testing Market is estimated to value over USD 98 billion by 27 end with a CAGR of.
In vitro toxicity tests have been wellestablished to support the needs of highthroughput screening in new drug development Invitrocue offers single dose and repeated dose toxicity tests that conform to regulatory guidelines for your R&D needs Dose finding (LD50). Invitro toxicology testing is defined as the cultured bacteria being affected by the chemical substances These methods are carried to identify dangerous chemicals to confirm the lack of assured contaminated properties in the initial stages of the growth of new substances which include food additives, therapeutic drugs, and agricultural chemicals. Acute inhalation toxicity testing for regulatory purposes is currently performed only in rats and/or mice according to OECD TG403, TG436, and TG433 test guidelines Such tests are biased by the differences in the respiratory tract architecture and function across species, making it difficult to draw conclusions on the potential hazard of inhaled compounds in humans.
The InVitro Toxicology and Toxicity Testing Market Report provides data on InVitro Toxicology and Toxicity Testing patterns and improvements, and target business sectors and materials, limits. The in vitro method is mainly used to elucidate mechanisms of toxicity in a drug and its effect on the cell or tissue The development of Ames Mutagenic Assay made the nonanimal testing more popular as compared to animal testing Furthermore, the regulatory concerns and political barriers are further expected to boost this market. In vitro toxicity testing provides useful data information to clarify toxicity generation and its mechanism and enables to save the time by eliminating toxicological elements in the early phase of drug discovery process In vitro toxicity testing allows for potential optimization of the concentration ranges in regards to toxic doses.
VitroScreen (Milan, Italy) GLP certified laboratory for in Vitro toxicology and in Vitro preclinical testing strategies skin irritation and corrosion (OECD 431 and 439), Absorption and penetration studies on human explants and 3D reconstructed skin and mucosae (OECD 428), Phototoxicity (OECD 432), Eye irritation (OECD 491 and 492), Skin sensitization (OECD 442D and 442E), Genotoxicity on FTskin and Biocompatibility for MD on 3D human tissue models. These in vitro model systems used in toxicity testing have diverse advantages together with the decrease in the number of animals, the reduced price of animal maintenance, as well as small amount. The advantage of cellbased toxicity assays is that large numbers of experiments can be conducted to screen the exponential dosetime combinations of exposure 3 It is greatly time and cost effective as compared to in vivo testing And ethical concerns of animal sacrifice and need for elaborate and regulated laboratories are avoided.
Jan , 21 (The Expresswire) Global “InVitro Toxicology Testing Market” forecast report delivers important insights and provides a complete analysis of. The high usage of toxicology testing in pharmacokinetic analysis of novel and generic modified pharmaceutical products results in the revenue growth of these toxicity testing assays Moreover, the presence of several drug candidates in the pipeline is expected to drive the demand for invitro toxicology testing. There is an urgent need to develop in vitro assays that correlate with in vivo toxicity tests in the study of venoms and in the assessment of the neutralizing ability of antivenoms, along with the 3Rs paradigm This goal must be strengthened by research funding agencies and agendas, regulatory agencies and diverse stakeholders related to.

Toxicity Tests Alternative Methods In Toxicology Prof Dimitrios Kouretas Ppt Download
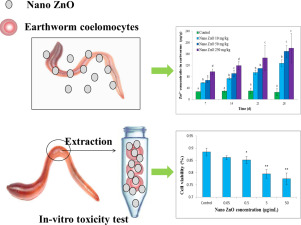
In Vivo And In Vitro Tests To Assess Toxic Mechanisms Of Nano Zno To Earthworms Science Of The Total Environment X Mol

In Vitro Toxicology Toxicity Testing Market Global Opportunity Analysis And Industry Forecast 19 25 Meticulous Market Research Pvt Ltd
In Vitro Toxicity Testing のギャラリー

In Vitro Toxicology Testing

In Vitro Toxicity Testing In Drug Development

In Vitro Toxicity Testing Youtube
Plos One Comprehensive In Vitro Toxicity Testing Of A Panel Of Representative Oxide Nanomaterials First Steps Towards An Intelligent Testing Strategy

In Vitro Toxicity Testing

Toxtutor Testing For And Assessing Toxicity
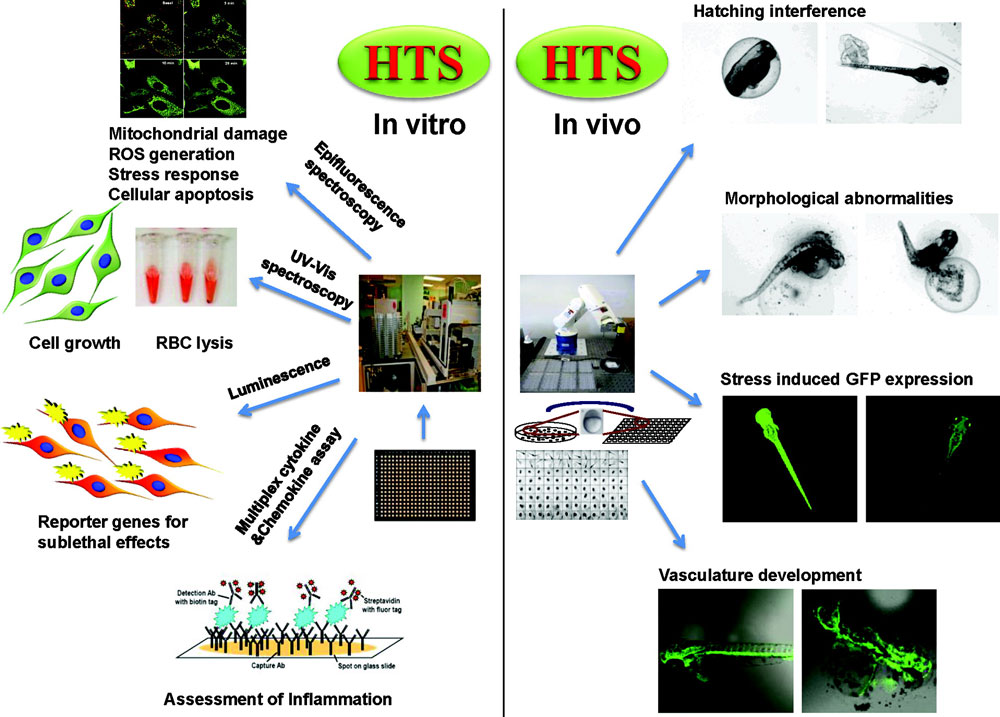
Nanolei Law And Nano Towards High Throughput Mechanisms Based Toxicity Testing Of Nanomaterials

Toxicity Testing In The 21st Century The Vision And Some Questions Kim Boekelheide Md Phd Brown University Society Of Toxicology Salt Lake City Ut Ppt Download

Transgenic Mouse Models Transferred Into The Test Tube New Perspectives For Developmental Toxicity Testing In Vitro Trends In Pharmacological Sciences

In Vitro Toxicology And Toxicity Testing Market To See Cagr Of 9 3 Percent

In Vitro Toxicity Testing Market Insights 19 Global And Chinese Analysis And Forecast To 24 z Press Release

In Vitro Toxicology Toxicity Testing Market To Grow At A Cagr Of 9 From 19 To Reach 14 4 Billion By 25 Meticulous Research Marketersmedia Press Release Distribution Services News Release Distribution Services

In Vitro Toxicity Testing

In Vitro Toxicity Testing Technologies And Global Markets

In Vitro Toxicity Testing Market Overview With Top Key Players

In Vitro Toxicity Studies Cellsystems Gmbh

Global In Vitro Toxicology And Toxicity Testing Market Industry Outlook Ge Healthcare Catalent Agilent Technologies Thermo Fisher Scientific The Daily Chronicle

In Vitro Toxicity Testing Of Environmental Agents Buy In Vitro Toxicity Testing Of Environmental Agents Online At Low Price In India On Snapdeal
Plos One Comprehensive In Vitro Toxicity Testing Of A Panel Of Representative Oxide Nanomaterials First Steps Towards An Intelligent Testing Strategy

Frequently Used Metabolizing Systems For In Vitro Toxicity Tests Download Table

Global In Vitro Toxicity Testing In Chemical Market By Onkar Nimbalkar Issuu
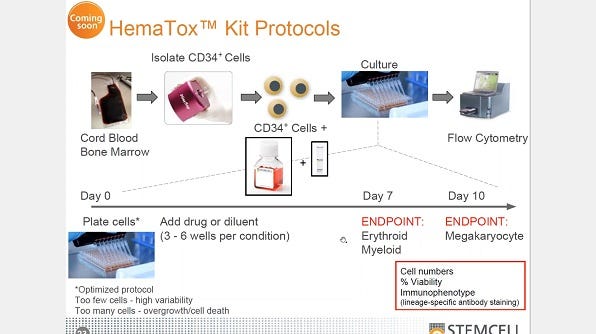
Tech Tips Protocols Drug Discovery And Toxicity Testing Areas Of Interest Scientific Resources
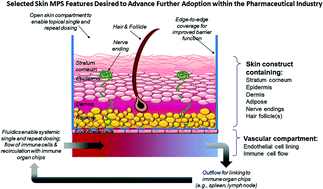
Drug Induced Skin Toxicity Gaps In Preclinical Testing Cascade As Opportunities For Complex In Vitro Models And Assays Lab On A Chip Rsc Publishing
In Vitro Toxicology

Global In Vitro Toxicology Testing Market Industry Research By Marketdataforecast Com By Lokesh Issuu

Environmental Factor September 07 Committee Proposes Paradigm Shift In Toxicity Testing
In Vitro Toxicology Toxicity Testing Market Size Share 17 27

In Vitro Toxicology Toxicity Testing Market Worth Usd 14 4 Billion By 25 In Vitro Toxicology Toxicity Testing Market Worth 14 4 Billion By 25 Pipettes Research Companies Marketing
Global In Vitro Toxicity Testing Market 19 23 Evolving Opportunities With Abbott Laboratories And Agilent Technologies Inc Technavio Business Wire
In Vitro Testing Of Drug Toxicity

In Vitro Toxicity Testing Market Is Expected To Reach 7 813

Global In Vitro Toxicology Testing Market 16 21 Increasing Drug Discovery And Innovation Modernization In The In Vitro Toxicity Testing Market Research And Markets

Part 2 Toxicity In Vitro Toxicity Studies Dōterra Essential Oils

In Vitro Toxicology Screening In Drug Development Admescope

In Vitro Toxicity Testing Market Is Seeing Explosive Growth By Future Industry Winners Forecast 14 22 Medgadget
In Vitro Toxicology

Expert Insights On In Vitro Alternatives For Drug And Chemical Toxicity Testing Eurekalert Science News

In Silico Toxicology Computational Methods For The Prediction Of Chemical Toxicity Raies 16 Wires Computational Molecular Science Wiley Online Library

In Vitro Toxicity Testing In Chemical Market Merck Kgaa

A Colloidal Singularity Reveals The Crucial Role Of Colloidal Stability For Nanomaterials In Vitro Toxicity Testing Nzvi Microalgae Colloidal System As A Case Study Kindle Edition By National Institutes Of Health Professional
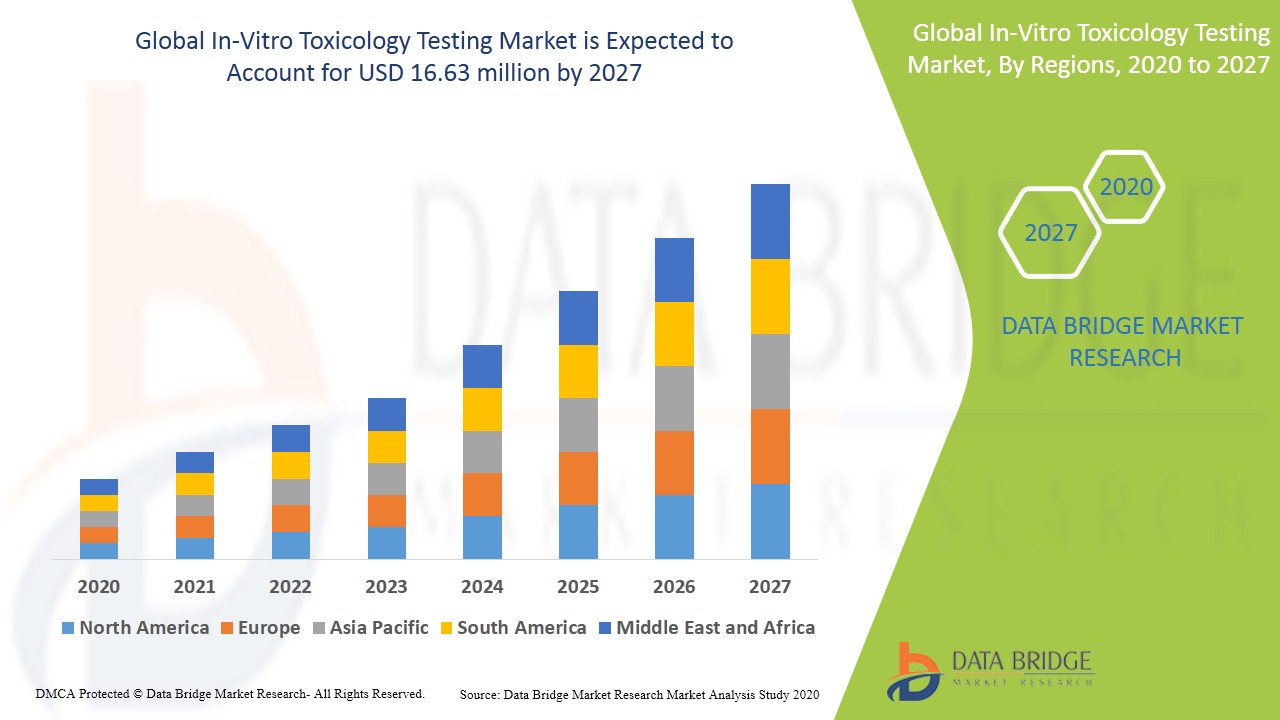
In Vitro Toxicology Testing Market Global Industry Trends And Forecast To 27 Data Bridge Market Research
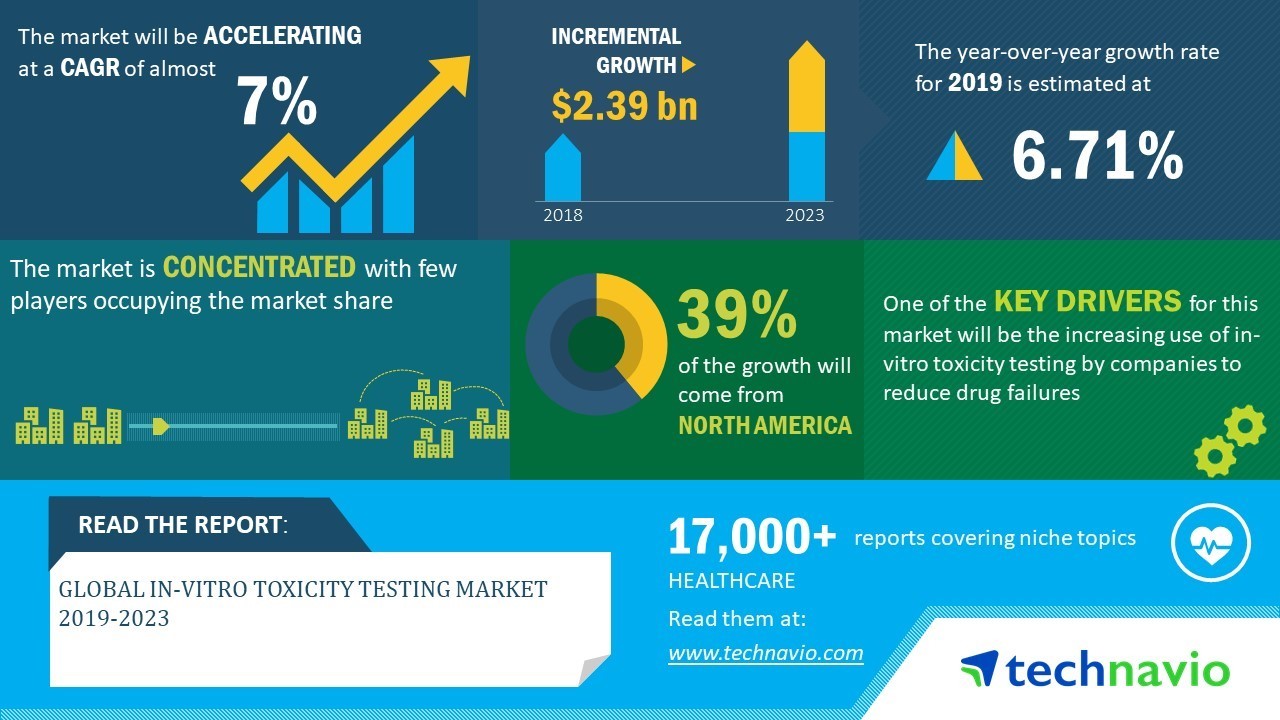
Global In Vitro Toxicity Testing Market 19 23 Evolving Opportunities With Abbott Laboratories And Agilent Technologies Inc Technavio Business Wire
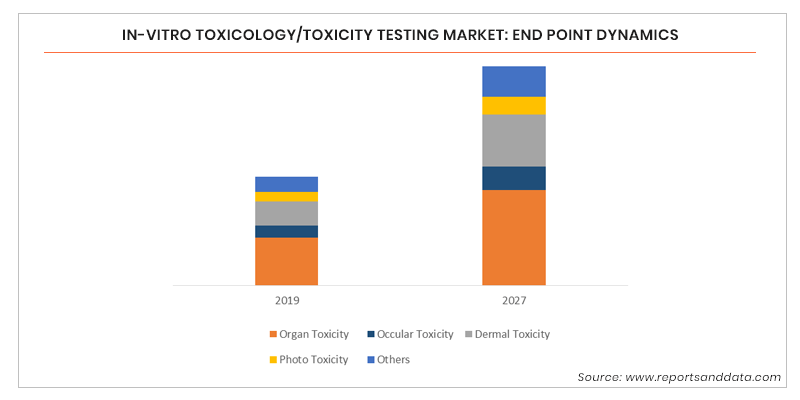
In Vitro Toxicology Toxicity Testing Market Size Share 17 27

In Vitro Toxicology Toxicity Testing Market Marketing Market Design Health Care
Global Market For In Vitro Toxicity Testing To Be Worth 2 7 Billion In 15

Models And Methods For In Vitro Toxicity Sciencedirect

In Vitro And In Vivo Toxicity Studies Of Copper Sulfide Nanoplates For Potential Photothermal Applications Sciencedirect

In Vitro Toxicity Testing Market The Daily Chronicle
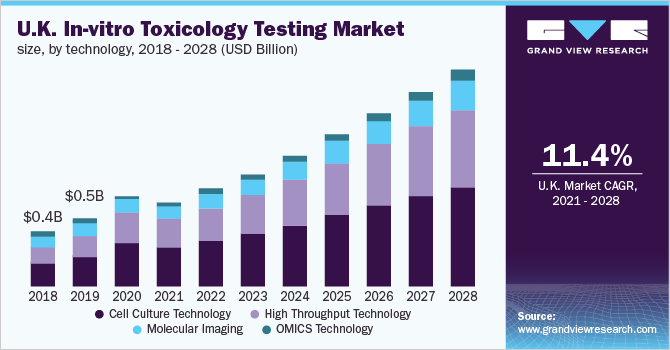
In Vitro Toxicology Testing Market Size Industry Report 27

Pdf High Content Screening For In Vitro Toxicity Testing

In Vitro Toxicity Testing Of Environmental Agents Springerlink
Plos One Comprehensive In Vitro Toxicity Testing Of A Panel Of Representative Oxide Nanomaterials First Steps Towards An Intelligent Testing Strategy

Databases To Help Computational Toxicology And In Vitro Toxicity Download Scientific Diagram

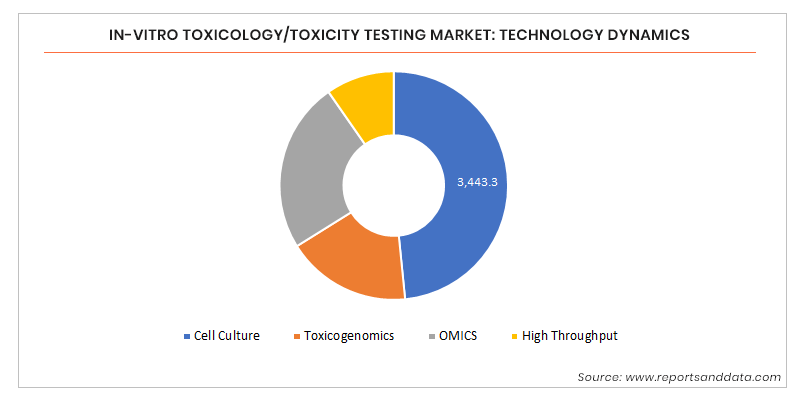
In Vitro Toxicology Toxicity Testing Market By Product And Service Technology Cell Culture Omics Method Cell Based Assays In Silico End Point Adme Genotoxicity Organ Toxicity Dermal Toxicity End User And Geography Global Forecast To 25
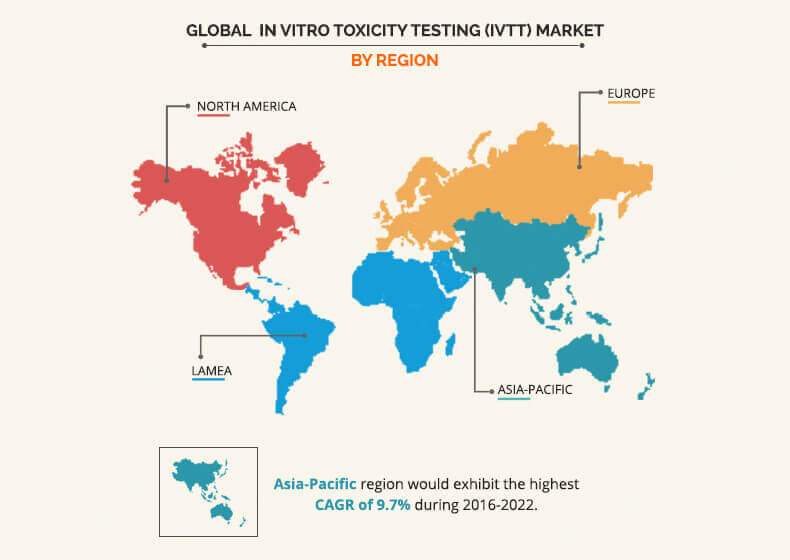
In Vitro Toxicity Testing Market By Product Type Industry 22
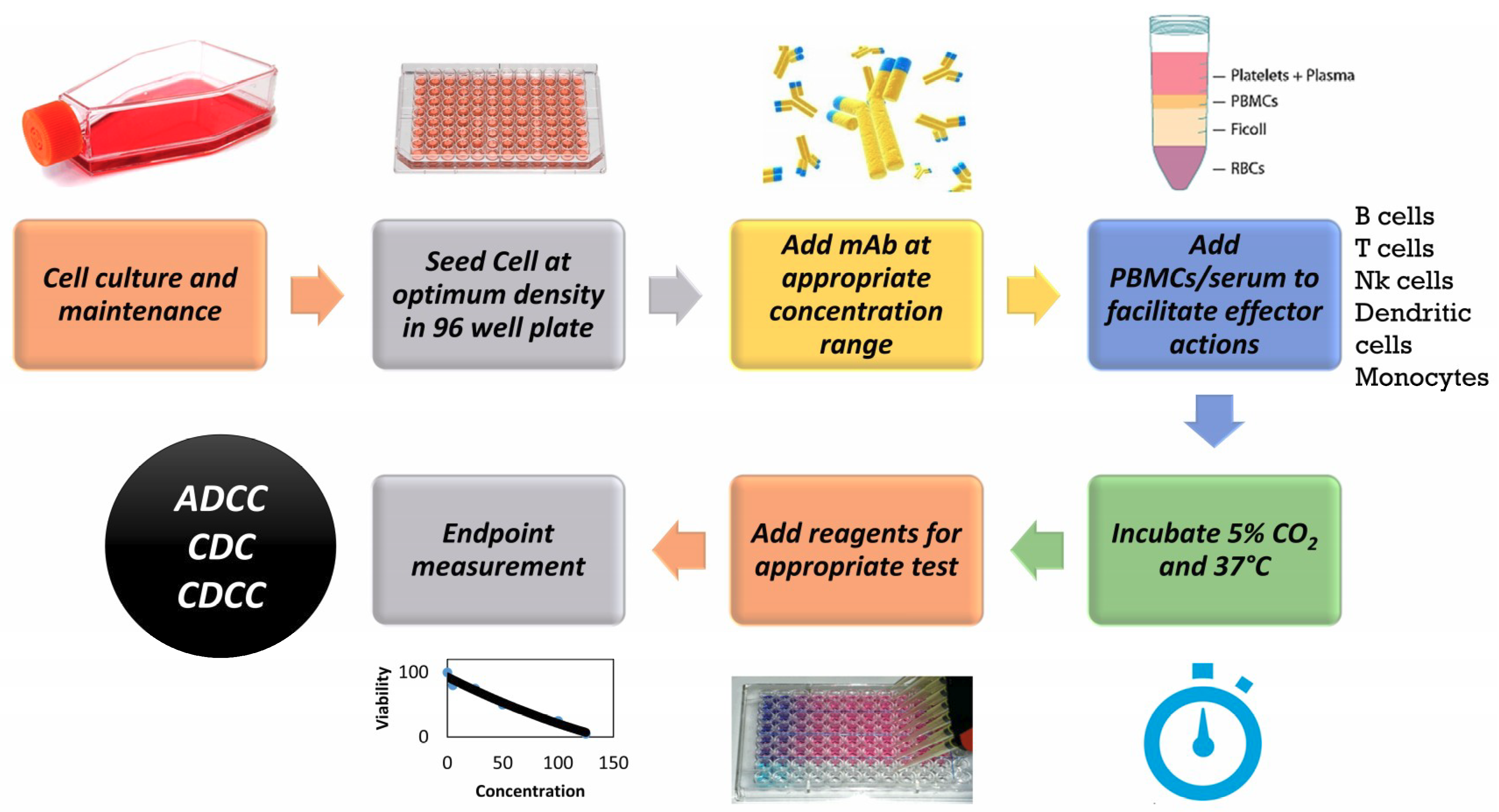
Antibodies Free Full Text Applicability Of Traditional In Vitro Toxicity Tests For Assessing Adverse Effects Of Monoclonal Antibodies A Case Study Of Rituximab And Trastuzumab

In Vitro Toxicology Testing The Barriers To Progression And Adoption Technology Networks

Porsolt

Toxicological Testing In Vivo And In Vitro Models Sciencedirect

Ppt Promising In Vitro Toxicity Testing Market Moves To Next Stage Powerpoint Presentation Id

In Vitro Testing Of Drug Toxicity

Principles For In Vitro Toxicology Sciencedirect

In Vitro Toxicity Testing Market Global Forecast To 25 Marketsandmarkets

In Vitro Toxicity Testing Market By Product Assay Western Blot Tissue Culture Equipment Assay Reagent Software Toxicity Endpoints Adme Skin Irritation Corrosion Industry Pharmaceutical Cosmetics Covid 19 Impact Global Forecast To 25

In Vitro Toxicity Testing Applications To Safety Evaluation Medicine Health Science Books Amazon Com
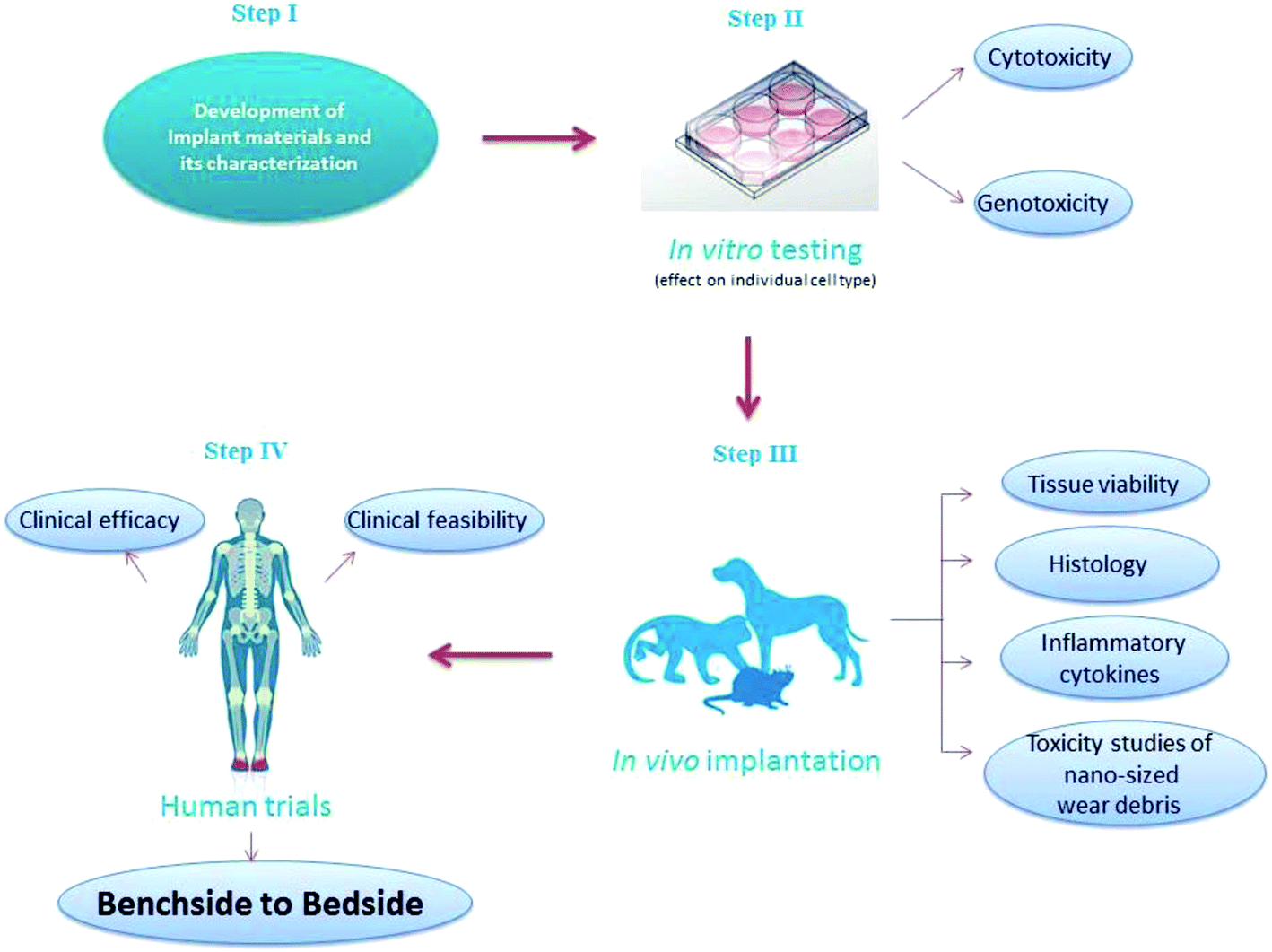
In Vitro In Vivo Assessment And Mechanisms Of Toxicity Of Bioceramic Materials And Its Wear Particulates Rsc Advances Rsc Publishing Doi 10 1039 C3ra444j
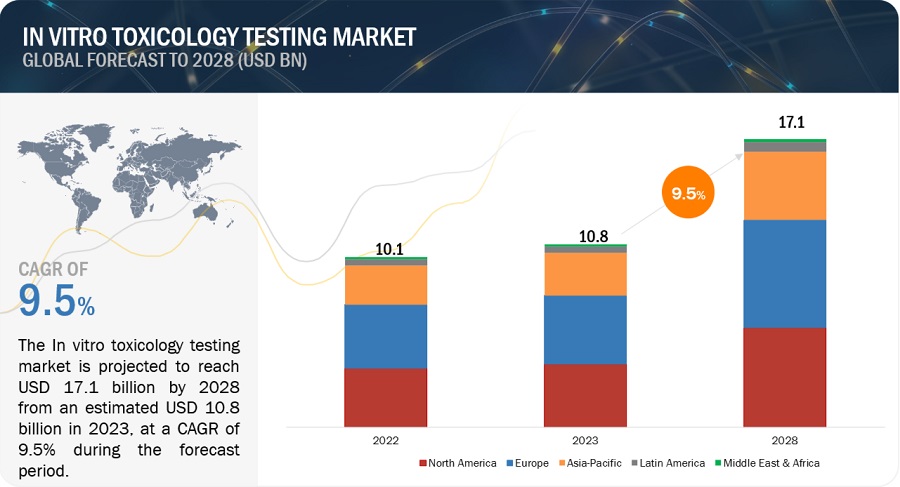
In Vitro Toxicity Testing Market Global Forecast To 25 Marketsandmarkets

Aerosol Exposure Devices For In Vitro Toxicity Testing At The Ali Youtube

In Vitro Toxicity Testing Market Size Growth Trends Industry Analysis Forecast Technavio
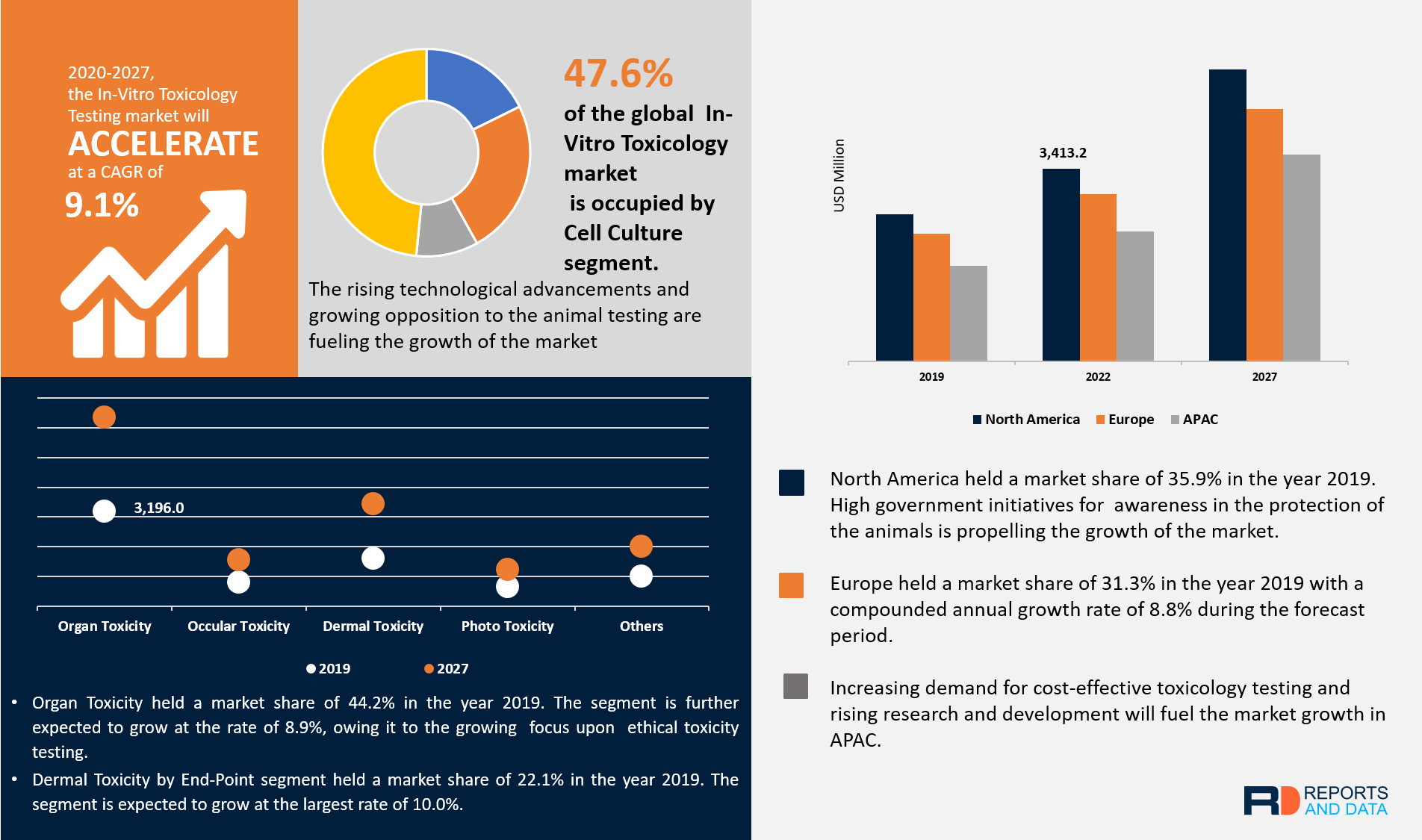
In Vitro Toxicology Toxicity Testing Market Size Share 17 27

Trinova Biochem Hemogenix Sup Sup Provides Assays For In Vitro Toxicity Screening Based On The Determination Of The Intracellular Atp Concentration

In Vitro Toxicology Wikipedia
2 Animal And In Vitro Toxicity Testing Toxicity Testing For Assessment Of Environmental Agents Interim Report The National Academies Press

In Vitro Toxicity Testing Market Size Trends Shares Insights And Forecast 27

In Vitro Toxicity Testing Market Size Share Development

Pdf Stem Cells And Predictive In Vitro Toxicity Testing Semantic Scholar
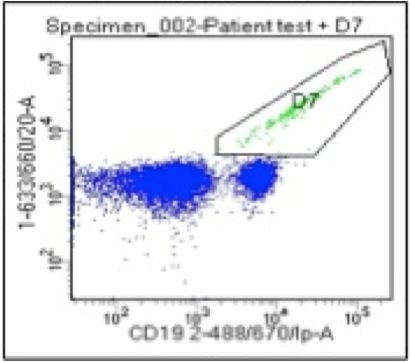
Drug Discovery In Vitro Toxicity Testing By Flow Cytometry Usa
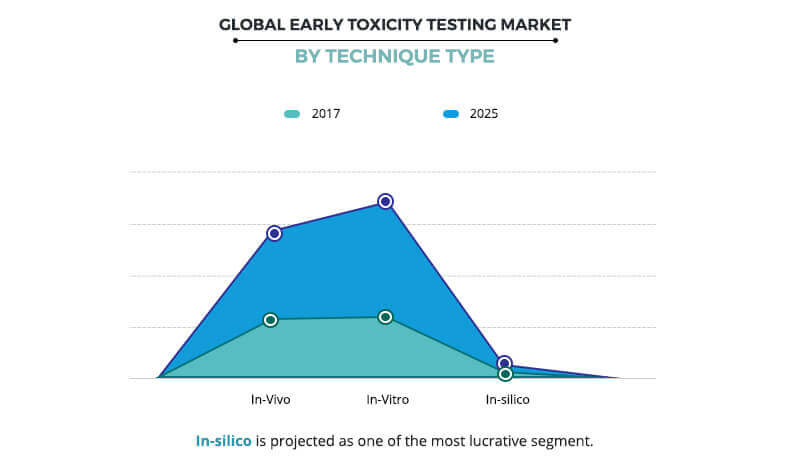
Early Toxicity Testing Market Size Share And Industry Analysis 25

Pdf 3 R Principle And Alternative Toxicity Testing Methods Semantic Scholar
Plos One Comprehensive In Vitro Toxicity Testing Of A Panel Of Representative Oxide Nanomaterials First Steps Towards An Intelligent Testing Strategy

In Vitro Toxicity Testing

Toxicological Tests Of Nanoparticles In Vitro Toxicity Download Table
In Vitro Toxicity Testing Market Global Forecast To 25 Marketsandmarkets

In Vitro Toxicology Testing Market Trends Volume Price Market Share By Company And By Location Average Price And Price Of Each Company
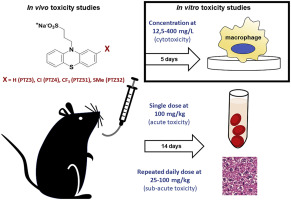
In Vitro And In Vivo Toxicity Evaluation Of Non Neuroleptic Phenothiazines Antitubercular Drug Candidates Regulatory Toxicology And Pharmacology X Mol

Global In Vitro Toxicity Testing Market To Reach 8 8bn By 23
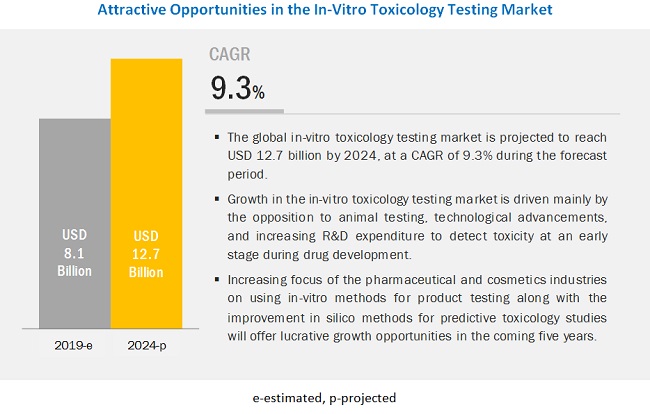
In Vitro Toxicology Testing Market Worth 12 7 Billion By 24 Icrowdnewswire

In Vitro Toxicity Testing Market

In Vitro Toxicity Testing Market Estimated To Grow At Cagr 15 06
In Vitro Toxicity Test Creative Biolabs

In Vitro Toxicity An Overview Sciencedirect Topics

Global In Vitro Toxicity Testing Market Size Trends Analysis With Covid 19 Impact By Yash Jain Issuu

In Vitro Toxicity Testing

In Vitro Toxicity Tests A Hemolysis Caused By Amb Na Upon Their Download Scientific Diagram

Microtissues For In Vitro Toxicity Assessment Cost Effective And In Vivo Relevant Toxicology Tools Drug Discovery World Ddw

In Vitro Toxicity Testing Protocols Springerlink

In Vitro Toxicology Charles River



