In Vitro Toxicity Testing Methods
Considering the ethical issues and the cost of invivo animal tests, the pharmaceutical industry now relies more on invitro methods for toxicity testing in the drug development phase Here, we answer the common questions regarding the invitro toxicity testing in drug development.
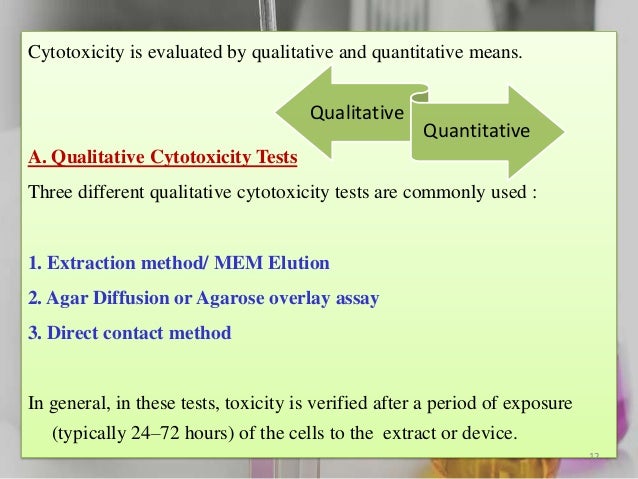
In vitro toxicity testing methods. The toxicity of any new compound can be assessed broadly in two ways (1) by using cells/cell. The US Tox21 collaborative program represents a paradigm shift in toxicity testing of chemical compounds from traditional in vivo tests to less expensive and higher throughput in vitro methods to prioritize compounds for further study, identify mechanisms of action and ultimately develop predictive models for adverse health effects in humans. For example, an Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) 3 has been formally established in US in 00, with the aim of (i) evaluating existing in vivo, in silico, and In vitro tests for acute systemic toxicity, and (ii) developing a strategic roadmap 4 where In vitro and in silico approaches enable.
Research Council (NRC) report, Toxicity Testing in the 21st Century A Vision and Strategy (NRC, 07), envisions a future in which toxicity testing relies primarily on the in vitro study of humanderived cells or cell lines Furthermore, the in vivo DARt methods used for regulatory purposes have. Global In Vitro Toxicity Testing in Chemical Market is expected to grow with a steady CAGR in the forecast period of 1926 The report contains data from the base year of 18 and the historic year of 17. The cells, agar and test sample are incubated for more than 24 hours and then examined under a microscope to determine the severity of toxicity to the cells based on a 04 grading scheme MEM Elution Unlike the agarose diffusion and direct contact methods, the MEM Elution procedure involves applying extractables and leachables from the test.
Invitro toxicology testing helps you to evaluate a product or ingredients’ potential to cause dermal/ocular irritation or dermal corrosion when used by a consumer The principal test methods are cellbased and biochemical assays, exvivo and insilico Acute Toxicity. Jan , 21 (The Expresswire) Global “InVitro Toxicology Testing Market” forecast report delivers important insights and provides a complete analysis of. In vitro systems are considered as alternative testing methods to reduce the use of animals in toxicity studies, refine toxicity evaluations (ie, to go beyond general growth or survival data), and replace in vivo studies—the socalled 3 Rs of alternative tests Cell or tissuebased approaches represent a more direct way to determine the mode of action of xenobiotics at the fundamental level.
In vitro Toxicology Safety Testing Services Test Guideline No Testing Price 3T3 NRU Cytotoxicity Study (an in vitro alternative to the animalbased in vivo LD50) Guidance Document No 129 RM 2, RM 2, (Packag Skin Corrosion In vitro EpiDerm Skin Corrosion (EPI0SCT) OECD 431 RM 5, Skin Irritation In vitro EpiDerm Skin. Concept of In Vitro Toxicity Tests This section will focus solely on in vitro methods for evaluating toxicity, as one of the alternatives to wholeanimal testing Additional nonanimal alternatives such as computer modelling and quantitative structureactivity relationships are discussed in other articles of this chapter. This has increased the acceptance of invitro toxicology and computational methods, consequently driving the market growth Growing ethical concerns regarding animal testing procedures and efforts taken by several animal welfare organizations have paved the way for the replacement or reduction of animal testing with invitro toxicology testing.
However, reliable in vitro testing methods to measure drug toxicity in the testis are lacking In recent years, in vitro toxicity models have reduced the use of experimental animals and saved. In Vitro Toxicity Testing Market Overview Global In Vitro Toxicity Testing(IVTT) Market was valued at $3,366 million in 16, and is expected to reach $7,813 million by 22, supported by a CAGR of 1501% during the forecast period 16 22 In vitro is the process that helps examine harmful chemicals over the isolated part of the organism. Invitro toxicology testing market size is expected to grow significantly from to 26Invitro toxicity testing involves the use of cultured cells or computer models to identify toxic or hazardous chemicals of new substances.
Toxicology studies that help to determine the toxicity profile of a new compound are a standard practice in the safety evaluation of new drugs and products Invitrocue can conduct standard in vitro and in vivo toxicology studies and work with clients to develop novel toxicology study designs. Research Council (NRC) report, Toxicity Testing in the 21st Century A Vision and Strategy (NRC, 07), envisions a future in which toxicity testing relies primarily on the in vitro study of humanderived cells or cell lines Furthermore, the in vivo DARt methods used for regulatory purposes have. In vitro Toxicology Safety Testing Services Test Guideline No Testing Price 3T3 NRU Cytotoxicity Study (an in vitro alternative to the animalbased in vivo LD50) Guidance Document No 129 RM 2, RM 2, (Packag Skin Corrosion In vitro EpiDerm Skin Corrosion (EPI0SCT) OECD 431 RM 5, Skin Irritation In vitro EpiDerm Skin.
MB Research Labs works closely with other leaders in the field of in vitro toxicology and is dedicated to advancing techniques that reduce the use of live animals in Toxicological Research Below are several related sources that are helpful in developing and validating novel in vitro toxicology testing methods National Toxicology Program. In vitro, ex vivo, and in silico testing methods are some of the alternatives to animal testing and are used to determine the toxicity of a substance Safety assessment and efficacy testing is a mandatory procedure for industries such as chemicals, pesticides, cosmetics, consumer products, drugs, vaccines, and medical devices. In Section 5 (Toxicity Testing Methods) Did a change in the title of the "Future Approaches and Methods" to "Emerging Approaches and Methods" Added additional content in "Finding Information about Alternatives to Animal Testing" Added Microphysiological Systems as used in "tissue chip" and "Organsonchips" models Added "HumanonaChip.
In Vitro Toxicity Testing Market Overview Global In Vitro Toxicity Testing(IVTT) Market was valued at $3,366 million in 16, and is expected to reach $7,813 million by 22, supported by a CAGR of 1501% during the forecast period 16 22 In vitro is the process that helps examine harmful chemicals over the isolated part of the organism. The cells, agar and test sample are incubated for more than 24 hours and then examined under a microscope to determine the severity of toxicity to the cells based on a 04 grading scheme MEM Elution Unlike the agarose diffusion and direct contact methods, the MEM Elution procedure involves applying extractables and leachables from the test. For In Vitro Toxicology Internationally recognized as a leader in the in vitro toxicology field, IIVS couples rigorous scientific and compliance programs with education and outreach initiatives to promote the use and acceptance of these methods worldwide.
InVitro Testing Alternative Methods to Assess Toxicology and Efficacy of Cosmetics InVitro in the Cosmetics Industry In the cosmetics industry, invitro testing is used to confirm the lack of certain EU Ban on Animal Testing In 13, EU Cosmetic Regulation (1223/09) introduced a ban on. Since in vitro toxicity testing methodology is evolving at such a rapid rate, this book can of necessity provide only a "snapshot" of the major techniques in use at the present time As with the other publications in Humana Press' Methods in Molecular Biology series, the aim has been to supply information on the fundamental requirements for the. With the development of in vitro, ex vivo and in silica toxicity evaluation methodologies, it has become apparent that these alternative methods can provide data as predictive and accurate, if not more, as in vivo testing.
Harvard’s Wyss Institute has created “organsonchips” that contain human cells grown in a stateoftheart system to mimic the structure and function of human organs and organ systems The chips can be used instead of animals in disease research, drug testing, and toxicity testing and have been shown to replicate human physiology, diseases, and drug responses more accurately than crude. In vitro toxicity testing protocols METHODS IN MOLECULAR BIOLOGY, HUMANA PRESS, TOTOWA, NJ (USA), 1995, vol 43, 332 pp. The US Tox21 collaborative program represents a paradigm shift in toxicity testing of chemical compounds from traditional in vivo tests to less expensive and higher throughput in vitro methods to prioritize compounds for further study, identify mechanisms of action and ultimately develop predictive models for adverse health effects in humans.
Jan , 21 (The Expresswire) Global “InVitro Toxicology Testing Market” forecast report delivers important insights and provides a complete analysis of. Understanding In Vitro and In Vivo Toxicology Testing for Chemicals Little Pro on. Considering the ethical issues and the cost of invivo animal tests, the pharmaceutical industry now relies more on invitro methods for toxicity testing in the drug development phase Here, we answer the common questions regarding the invitro toxicity testing in drug development.
Drug toxicity testing Preclinical in vitro and in vivo toxicology evaluation is a critical step in pharmaceutical drug development Unexpected issues with ADME or toxicity account for 5060% of drug development program failures Safety assessment issues and risks for humans are identified through hepatotoxicity, genotoxicity, immunogenicity. Animal testing methods, our scientists conduct a broad range of invitro testing services for cosmetic products These address the issues of skin and eye irritation, skin corrosion, cytotoxicity, phototoxicity and mutagenicity Using stateoftheart testing equipment, our invitro toxicology testing labs deliver testing services that comply with. Cosmetic, Beauty, Healthcare Products Testing MB Research has been conducting product safety assessments for the cosmetics, personal care, chemical and pharmaceutical industries for over 40 years and is often sought out by Cosmetics Industry Leaders to ensure the safety their ingredients and aid in the rapid development of products MB Research Scientists develop and conduct in vitro and.
The use of concentrations relative to. Concept of In Vitro Toxicity Tests This section will focus solely on in vitro methods for evaluating toxicity, as one of the alternatives to wholeanimal testing Additional nonanimal alternatives such as computer modelling and quantitative structureactivity relationships are discussed in other articles of this chapter. To identify key procedures based upon internationally recognized guidelines.
In vitro toxicity testing is the scientific analysis of the effects of toxic chemical substances on cultured bacteria or mammalian cells In vitro testing methods are employed primarily to identify potentially hazardous chemicals and/or to confirm the lack of certain toxic properties in the early stages of the development of potentially useful new substances such as therapeutic drugs, agricultural chemicals and food additives In vitro assays for xenobiotic toxicity are recently carefully consid. Integrated testing strategies combine methods, such as in silico methods and in vitro assays, along with appropriate statistical analysis, for the prediction of in vivo toxicity responses An understanding of the mechanisms of toxicity and the cellular pathways involved are currently being investigated with the goal of obtaining more predictive. Concept of In Vitro Toxicity Tests This section will focus solely on in vitro methods for evaluating toxicity, as one of the alternatives to wholeanimal testing Additional nonanimal alternatives such as computer modelling and quantitative structureactivity relationships are discussed in other articles of this chapter.
Toxicology studies that help to determine the toxicity profile of a new compound are a standard practice in the safety evaluation of new drugs and products Invitrocue can conduct standard in vitro and in vivo toxicology studies and work with clients to develop novel toxicology study designs. The CM test method was the first in vitro test method available in the US for this purpose ICCVAM concluded that the performance of the other four test methods required improvement before they could be used in regulatory safety testing to classify substances as having the potential to cause reversible, nonsevere eye injuries or as not. The CM test method was the first in vitro test method available in the US for this purpose ICCVAM concluded that the performance of the other four test methods required improvement before they could be used in regulatory safety testing to classify substances as having the potential to cause reversible, nonsevere eye injuries or as not.
Jan , 21 (The Expresswire) Global “InVitro Toxicology Testing Market” forecast report delivers important insights and provides a complete analysis of. Toxicology studies that help to determine the toxicity profile of a new compound are a standard practice in the safety evaluation of new drugs and products Invitrocue can conduct standard in vitro and in vivo toxicology studies and work with clients to develop novel toxicology study designs. This report will give a general overview of some of the in vitro methodologies used in toxicity testing The use of computerbased structureactivity relationships and cell culture testing systems can provide valuable toxicological data for hazard and risk assessments In vitro systems allow for a m.
Toxicology studies that help to determine the toxicity profile of a new compound are a standard practice in the safety evaluation of new drugs and products Invitrocue can conduct standard in vitro and in vivo toxicology studies and work with clients to develop novel toxicology study designs. In vitro Toxicology Safety Testing Services Test Guideline No Testing Price 3T3 NRU Cytotoxicity Study (an in vitro alternative to the animalbased in vivo LD50) Guidance Document No 129 RM 2, RM 2, (Packag Skin Corrosion In vitro EpiDerm Skin Corrosion (EPI0SCT) OECD 431 RM 5, Skin Irritation In vitro EpiDerm Skin. Suggested Citation"4 METHODS FOR TOXICITY TESTING"National Research Council 1993 Pesticides in the Diets of Infants and ChildrenWashington, DC The National Academies Press doi /2126.
Animal testing methods, our scientists conduct a broad range of invitro testing services for cosmetic products These address the issues of skin and eye irritation, skin corrosion, cytotoxicity, phototoxicity and mutagenicity Using stateoftheart testing equipment, our invitro toxicology testing labs deliver testing services that comply with. Jan , 21 (The Expresswire) Global “InVitro Toxicology Testing Market” forecast report delivers important insights and provides a complete analysis of. HumanSpecific Methods – The Challenges In vitro toxicity testing should build upon test models that are relevant for the species to be protected Proper test development requires well defined test compounds with high quality in vivo data (gold standard) and cell systems that mimic in vitro the key events that are known to occur in vivo Outside the pharmaceutical industry, adequate gold.
4 Validation of new methods is timeconsuming and expensive;. The In Vitro Toxicity Testing of Tobacco Smoke Task Force was established in 02 to ensure that CORESTA provides leadership on assessing toxicity evaluation The first phase was stated as follows To prepare a report covering the rationale and strategy for conducting in vitro toxicity testing of cigarette smoke;. In vitro assays bring a number of technical advantages to the conventional method of testing substances on animal models, as outlined, for example, in the 07 US National Research Council (NRC) report These advantages include the ability to elucidate cellularresponse networks and toxicity pathways;.
Concept of In Vitro Toxicity Tests This section will focus solely on in vitro methods for evaluating toxicity, as one of the alternatives to wholeanimal testing Additional nonanimal alternatives such as computer modelling and quantitative structureactivity relationships are discussed in other articles of this chapter. Acceptance of in vitro tests as alternatives to traditional toxicity testing in whole animals is expected to be slow 7 While many schemes. Today, the cosmetic industry has several fully validated in vitro testing methods that meet the regulatory requirements for sensitization assessment Toxicity Certain chemicals, if present in cosmetics products, even in very small doses, can cause temporary or permanent damage to human skin, eyes, and DNA.

In Vitro Testing Of Drug Toxicity

Toxtutor Testing For And Assessing Toxicity

Pdf 3 R Principle And Alternative Toxicity Testing Methods Semantic Scholar
In Vitro Toxicity Testing Methods のギャラリー

4 Tools And Technologies Toxicity Testing In The 21st Century A Vision And A Strategy The National Academies Press

In Vitro Toxicity Testing Protocols Methods In Molecular Biology 43 Medicine Health Science Books Amazon Com
In Vitro Toxicity Test Creative Biolabs

Novel In Vitro Approaches For Toxicity Testing Of Inhaled Substances Eurekalert Science News

In Vitro Toxicology Screening In Drug Development Admescope

How Are In Vitro Testing Methods Being Used In The Cosmetic Industry Invitrointl
Plos One Comprehensive In Vitro Toxicity Testing Of A Panel Of Representative Oxide Nanomaterials First Steps Towards An Intelligent Testing Strategy

Developing Context Appropriate Toxicity Testing Approaches Using New Alternative Methods Nams Altex Alternatives To Animal Experimentation

In Vitro Toxicity Testing Market

4 Tools And Technologies Toxicity Testing In The 21st Century A Vision And A Strategy The National Academies Press

In Vitro Toxicity Testing Market Global Forecast To 25 Marketsandmarkets

In Vitro Toxicity Testing

In Vitro Toxicology Toxicity Testing Market Global Opportunity Analysis And Industry Forecast 19 25 Meticulous Market Research Pvt Ltd
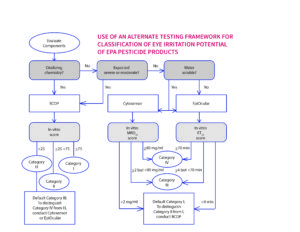
Momentum Grows For Modernizing The Us Epa Six Pack Battery Of Acute Toxicity Tests

In Vitro Toxicology Testing Market Size Share Trends Forecast
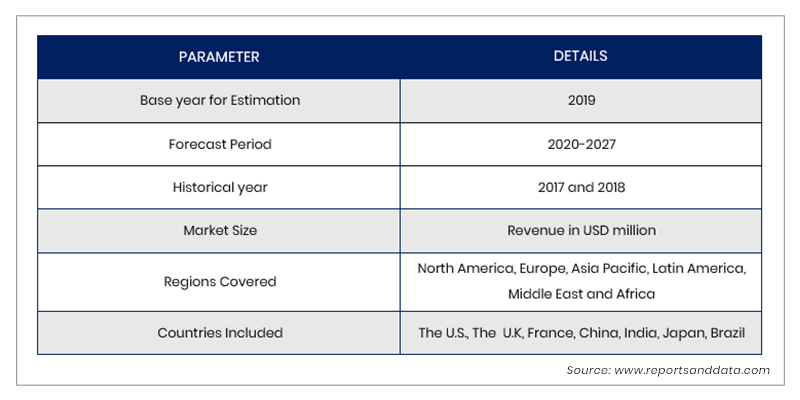
In Vitro Toxicology Toxicity Testing Market Size Share 17 27

Examples Of In Vitro And In Vivo Methods Specific Biological Download Scientific Diagram

The Dordick Research Group Artificial Intelligence And Machine Learning Aug 19 Rb

In Vitro Testing Of Drug Toxicity

Assessing The Human Health Risks Of Perfluorooctane Sulfonate By In Vivo And In Vitro Studies Sciencedirect

Toxtutor Testing For And Assessing Toxicity
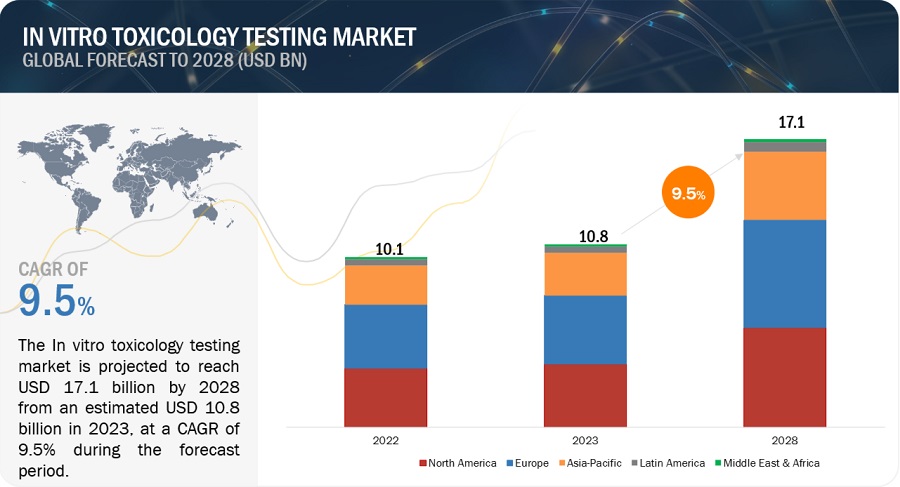
In Vitro Toxicity Testing Market Global Forecast To 25 Marketsandmarkets

In Vitro Toxicity Testing Protocols Springerlink

Ny Headline In Vitro Toxicology Toxicity Testing Market To Grow At A Cagr Of 9 From 19 To Reach 14 4 Billion By 25 Meticulous Research

Models And Methods For In Vitro Toxicity Sciencedirect

In Vitro Toxicology Toxicity Testing Market By Product And Service Technology Cell Culture Omics Method Cell Based Assays In Silico End Point Adme Genotoxicity Organ Toxicity Dermal Toxicity End User And Geography Global Forecast To 25
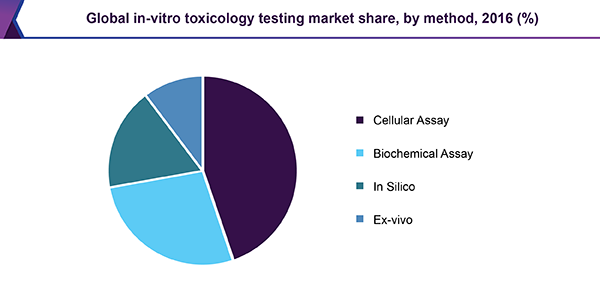
Latest Market Research Updates In Vitro Toxicology Testing Market Size Would Raise Around 26 98 Billion By 25

In Vitro Toxicology Testing Market Size Forecast Report 14 25 By Million Insights Research Analysis Issuu

Cell Culture Methods For In Vitro Toxicology Springerlink

Integrated Approaches To Testing And Assessment Iata Oecd

Toxtutor Testing For And Assessing Toxicity
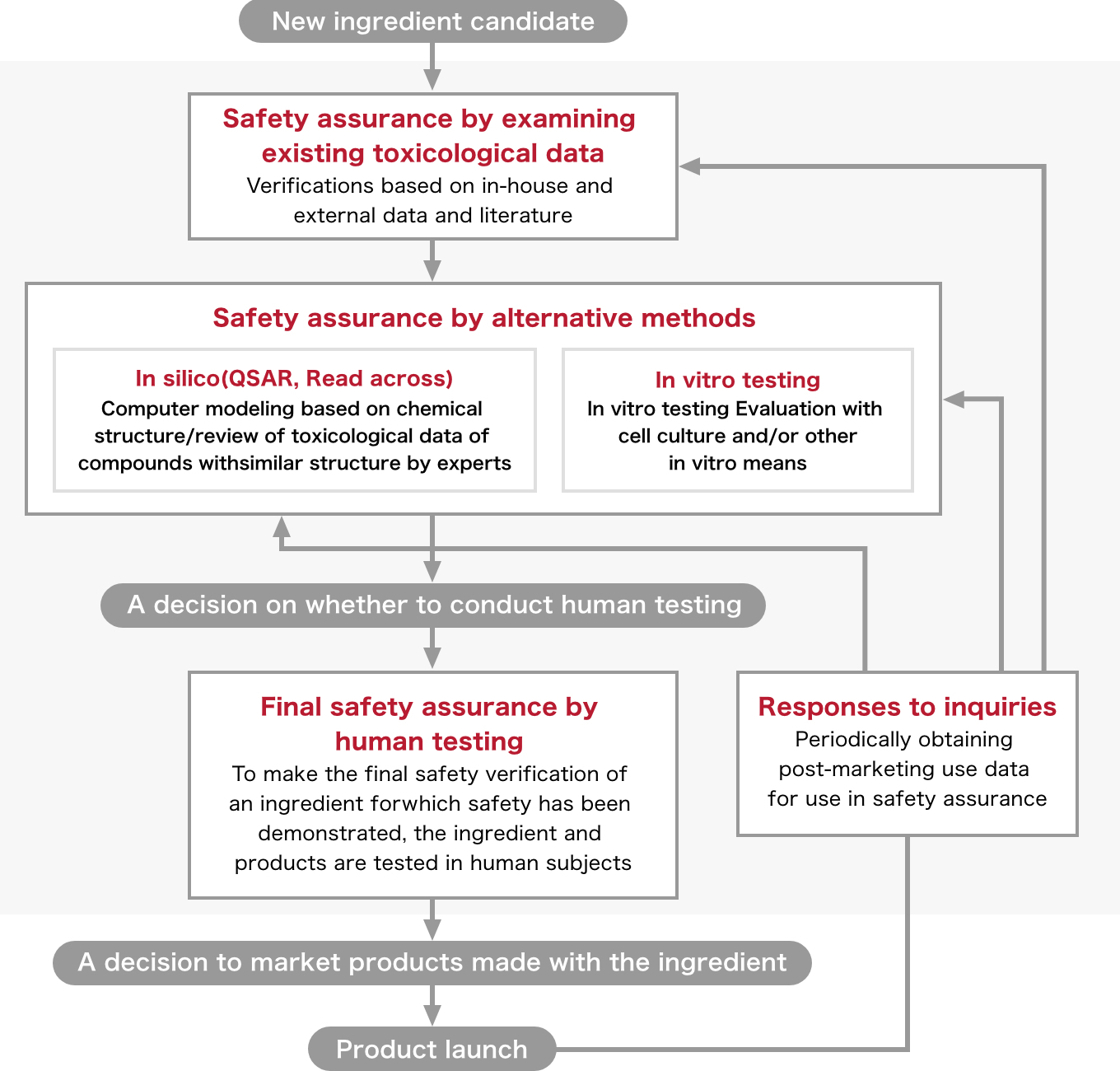
Shiseido Safety Assurance System Safety Assurance Innovation Shiseido Company

Assessing Toxicity With Human Cell Based In Vitro Methods Trends In Molecular Medicine

Top Pdf Toxicity Tests Methods 1library

Toxicity Tests Alternative Methods In Toxicology Prof Dimitrios Kouretas Ppt Download

In Vitro Toxicology Testing The Barriers To Progression And Adoption Technology Networks

In Vitro Toxicology Wikipedia

In Vitro Toxicology Market Youtube
In Vitro Toxicology

Full Text High Throughput Approaches For Genotoxicity Testing In Drug Developmen Ijhts

Announcements Altex Alternatives To Animal Experimentation

Recent Progress Of In Vitro Toxicity Assays In Drug Discovery

High Throughput Toxicity Screening And Intracellular Detection Of Nanomaterials Collins 17 Wires Nanomedicine And Nanobiotechnology Wiley Online Library

In Silico Toxicology Computational Methods For The Prediction Of Chemical Toxicity Raies 16 Wires Computational Molecular Science Wiley Online Library

Methods For Non Animal Testing Alttox Org

In Vitro Toxicity Testing In Drug Development

Global In Vitro Toxicity Testing In Chemical Market By Onkar Nimbalkar Issuu

In Vitro Toxicology Testing

In Vitro Toxicology 1st Edition
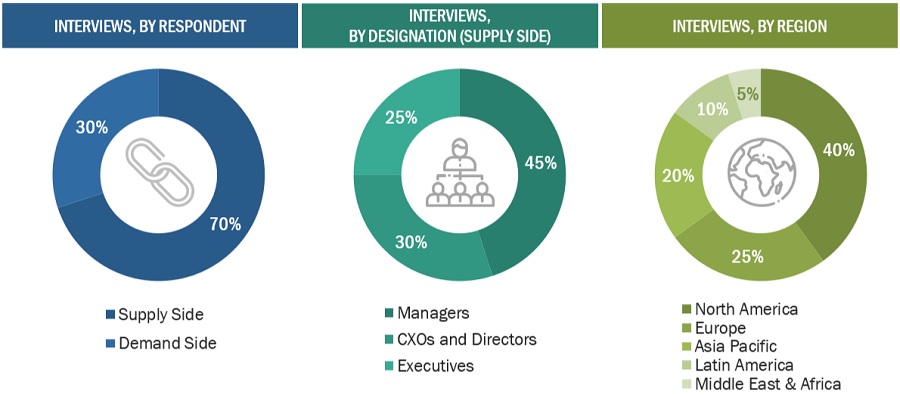
In Vitro Toxicity Testing Market Global Forecast To 25 Marketsandmarkets

Print Page

In Vitro Methods In Toxicology Medicine Health Science Books Amazon Com
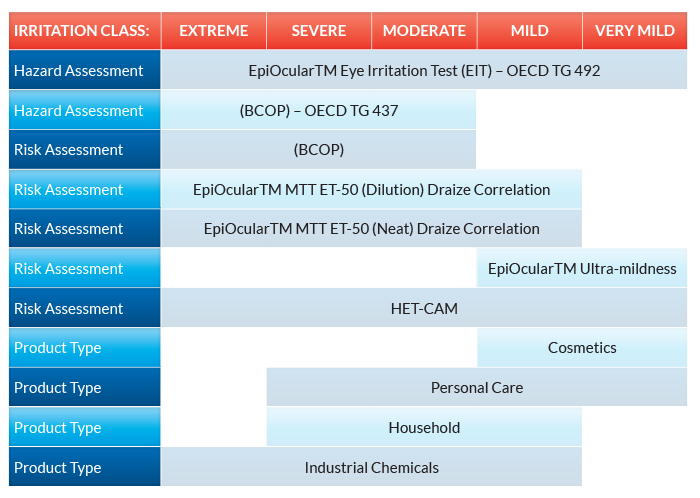
In Vitro Division Cpt Labs

Toxicity Testing An Overview Sciencedirect Topics

In Vitro Toxicology Testing Market Worth 17 227 Million By 18 By Billy Waterman Issuu

In Vitro Toxicology Testing Market Size Share Trends Analysis Report By Technology Cell Culture High Throughput By Method By Application By End Use By Region And Segment Forecasts 27
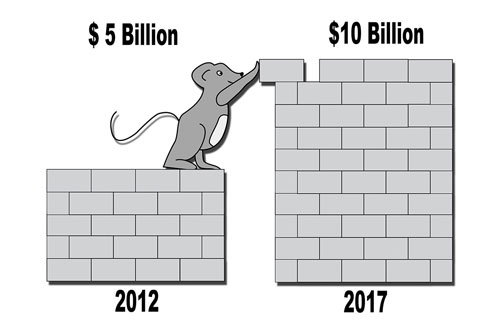
Alternatives To Animal Testing Drive Market

Toxicity Assessment Of Nanoparticles In Various Systems And Organs In Nanotechnology Reviews Volume 6 Issue 3 17

Toxicity Tests Alternative Methods In Toxicology Prof Dimitrios Kouretas Ppt Download

Robatt Replacement Ocular Battery

In Vitro Toxicology Systems Springerlink
Toxicity Testing In The 21st Century Defining New Risk Assessment Approaches Based On Perturbation Of Intracellular Toxicity Pathways
A Highly Quantitative In Vitro Method For Mechanistic Genotoxicity Screening Webinar Usa
Global In Vitro Toxicology Toxicity Testing Market 18 Video Dailymotion

Figure 1 From Methods Of In Vitro Toxicology Semantic Scholar

Transgenic Mouse Models Transferred Into The Test Tube New Perspectives For Developmental Toxicity Testing In Vitro Trends In Pharmacological Sciences

In Vitro Testing Of Drug Toxicity

Unifying The Effort Behind In Vitro Alternative Method Development Alttox Org

Aquatic Toxicology Wikipedia
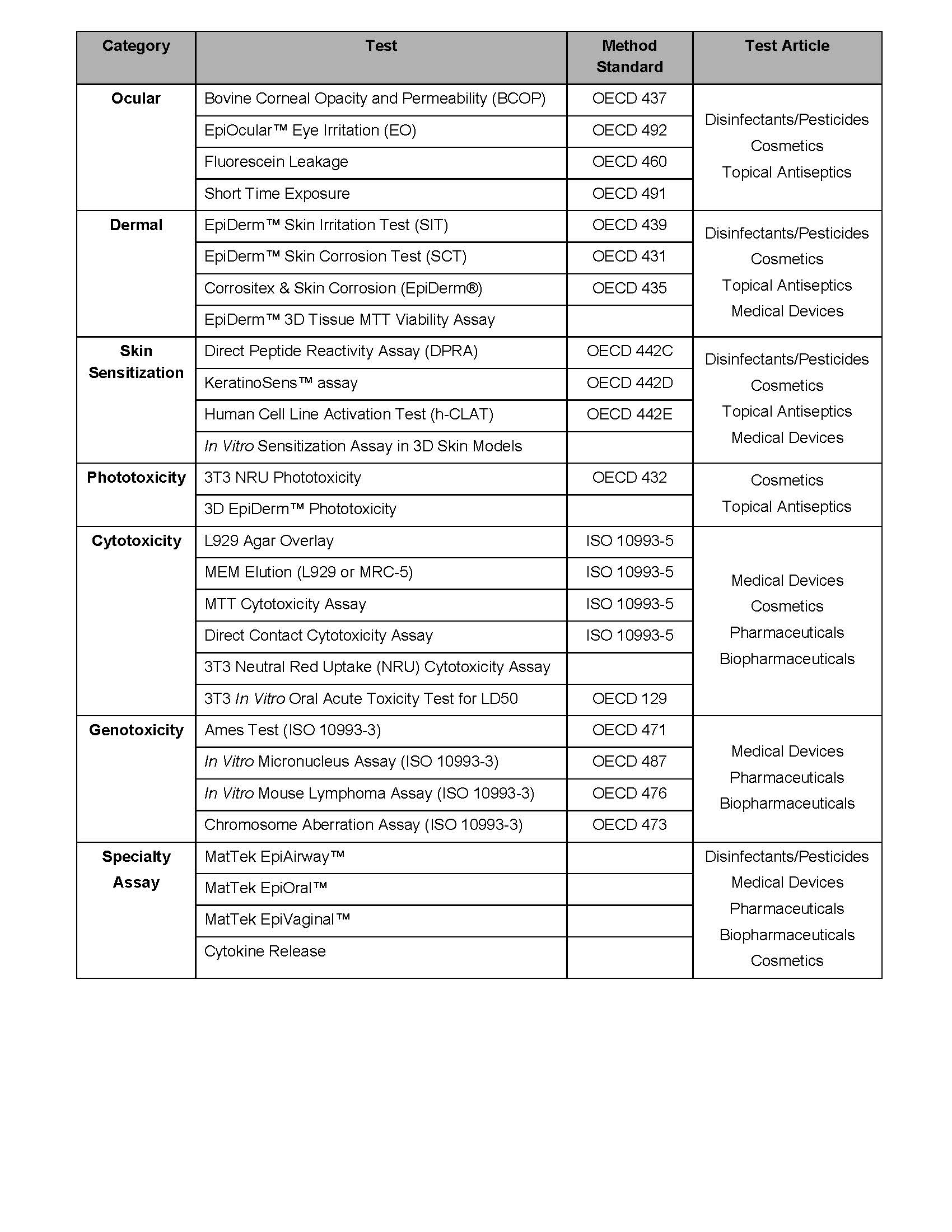
Toxicology Microbac Laboratories
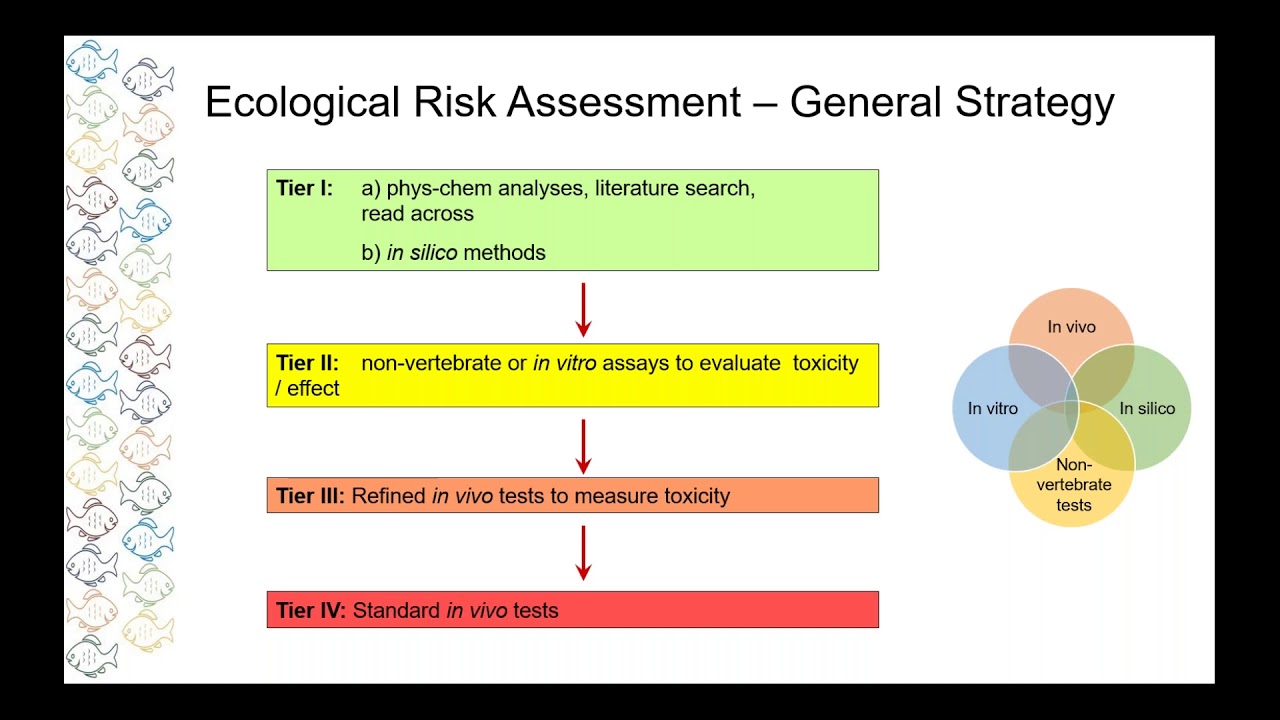
New Approaches For Fish Toxicity Testing Youtube

5 In Vivo And In Vitro Studies For Nanotoxicology Research Download Scientific Diagram

In Vitro Toxicity Testing

Consensus Report On The Future Of Animal Free Systemic Toxicity Testing Altex Alternatives To Animal Experimentation

Summary Of Eurl Ecvam Strategy To Replace Reduce And Refine The Use Of Download Scientific Diagram

Hazard Assessment Toxicity Testing Overview Alttox Org

Decision Tree Testing Strategy For Acute Environmental Toxicity Testing Download Scientific Diagram
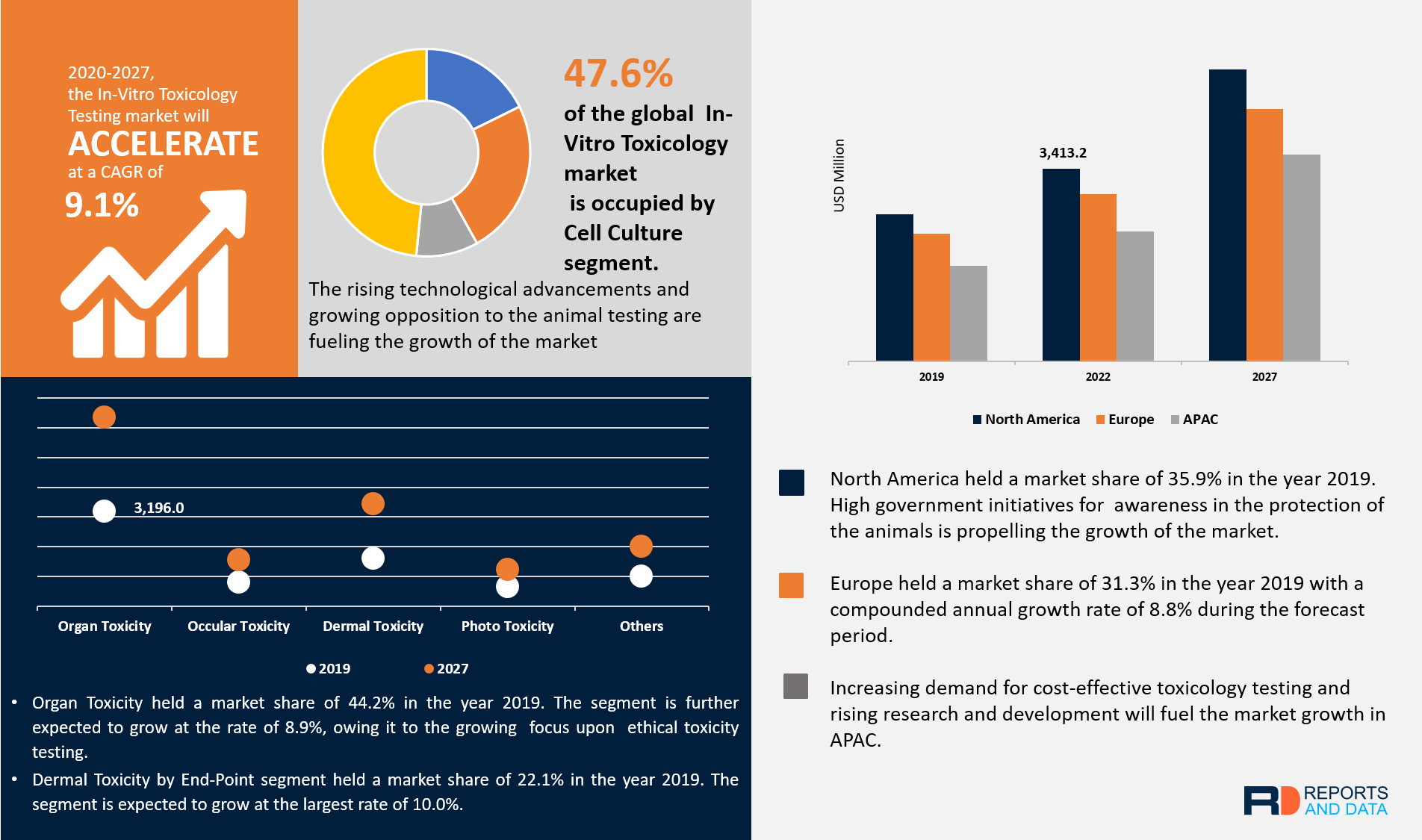
In Vitro Toxicology Toxicity Testing Market Size Share 17 27

Toxicity Tests Alternative Methods In Toxicology Prof Dimitrios Kouretas Ppt Download
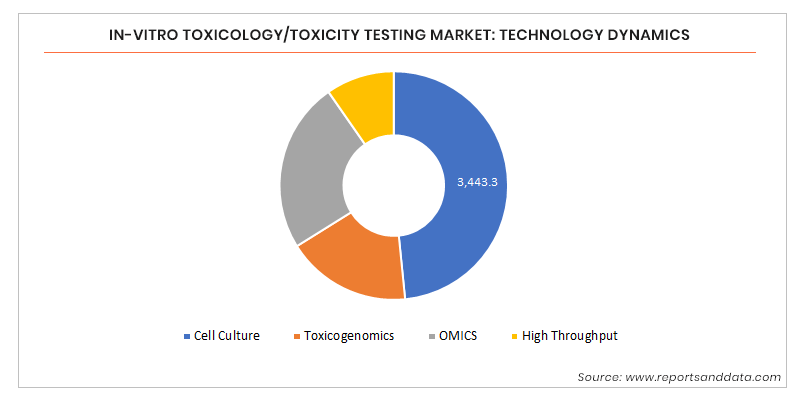
In Vitro Toxicology Toxicity Testing Market Size Share 17 27
Toxicity Testing In The 21st Century Defining New Risk Assessment Approaches Based On Perturbation Of Intracellular Toxicity Pathways

In Vitro Toxicology Testing

Principles For In Vitro Toxicology Sciencedirect
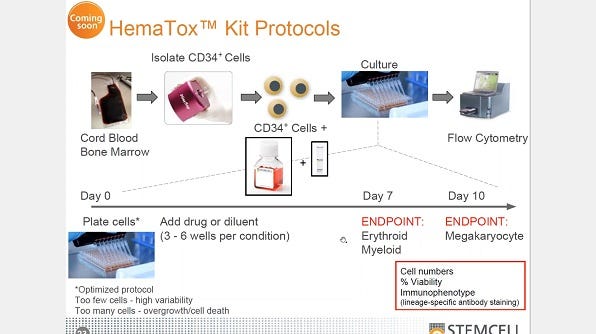
Tech Tips Protocols Drug Discovery And Toxicity Testing Areas Of Interest Scientific Resources

Troubleshooting Methods For Toxicity Testing Of Airborne Chemicals In Vitro Semantic Scholar



