In Vitro Testing Vs Animal Testing
‘In vitro testing’ is another excellent auxiliary to animal experimentation, in which cellular tests take place inside a test tube ‘Organs on chips’ is an innovative little device that helps scientists study the human biological system, drug metabolism, and diseases.
In vitro testing vs animal testing. This will decrease the use of animals in the testing process Furthermore, SC can show a specific organ’s reaction to a substance Nevertheless, in vitro techniques won’t show how the entire human system would respond Therefore, investigations with animals are still needed because animal models are being considered the most similar model to represent the human body Hence, these in vivo experiments will increase our knowledge and contribute to a better human but also a better animal. For instance although animal models provide some drawbacks like difference in biokinetics parameters or extrapolation of results to human, they are more reliable than in vitro tests The disadvantage of the in vitro procedures is that they are mostly performed on cancerous cell lines that have a substantially abnormal function. Considering the ethical issues and the cost of invivo animal tests, the pharmaceutical industry now relies more on invitro methods for toxicity testing in the drug development phase Here, we answer the common questions regarding the invitro toxicity testing in drug development.
Basically, the difference between in vivo and in vitro is that in vivo means that in cosmetic testing, the companies are testing on live animals However, in vitro testing draw from live cells and tissues, which means that no animals are being tortured and killed Going along with the 3 Rs, it uses both in vivo and in vitro testing. Indeed more than 70% of all in vitro assays captured by the survey were used since 10, peaking at just over 190,000 in 12 To be clear, reducing animal usage is not as simple as swapping out an animal assay for an in vitro alternative Alternatives can’t always tell us, for instance, if a compound will cause DNA damage leading to cancer. In vitro testing of cosmetic products is a widely available and costeffective alternative to animal testing It is also a powerful solution for driving product development, innovation and claim substantiation However, all research methods have their pros and cons, and in vitro testing is no exception In this article, we will examine the advantages and limitations of in vitro testing for.
Side a living organism and used for testing Although animals are still required as a source for these in vitro systems, the animal would experience distress for a much shorter time, and perhaps less distress overall, than occurs with wholeanimal testing because it would be killed before any experimental manipulations were carried out. Scientists often study the effects of drugs and chemicals on animals before they deem them safe for humans When possible, they try to perform these toxicology tests using biochemical or cellbased ( in vitro) systems instead of with animals such as mice. In vitro comes from the Latin term "in glass" The term refers to studies of biological properties that are done in a test tube (ie in a glass vessel) rather than in a human or animal In vitro studies are often contrasted to in vivo ("in life") studies which are done inside an organism.
In vitro testing occurs in a laboratory and usually involves studying microorganisms or human or animal cells in culture This methodology allows scientists to evaluate various biological. Animal rights groups have long propagated the myth that animal research could be replaced tomorrow by a plethora of "alternatives," from computer modeling and microdosing to MRI scanning and in vitro testing So, why the quotation marks on "alternatives"?. Though considerable advances have been made to develop in vitro and in silico technologies to test drug efficacy, toxicity and identify potential adverse reactions, the application of animal testing in the drug development process is still a necessity Current technology used to mimic the complexity of the human.
In vitro testing of cosmetic products is a widely available and costeffective alternative to animal testing It is also a powerful solution for driving product development, innovation and claim substantiation However, all research methods have their pros and cons, and in vitro testing is no exception In this article, we will examine the advantages and limitations of in vitro testing for. Basically, the difference between in vivo and in vitro is that in vivo means that in cosmetic testing, the companies are testing on live animals However, in vitro testing draw from live cells and tissues, which means that no animals are being tortured and killed Going along with the 3 Rs, it uses both in vivo and in vitro testing. The in silico method can be interpreted as testing that involves a computer or use of computer simulation in experimental research Common ways that in silico methods are incorporated into experiments are Planning experiments and power analysis computer tools are used to improve the design of in vivo and in vitro experiments.
The ethical dilemmas of animals testing are legitimate and deniable A second argument against animals testing is that there are alternative methods that can be used it similar experiments instead of animals In vitro testing and studying cell cultures can allow scientists to test on actual human cells There is a procedure called microdosing. Animal testing has, to date, of a wider move towards the integration of computer models for drug safety testing which includes the Comprehensive in vitro Proarrhythmia Assay initiative,. Though considerable advances have been made to develop in vitro and in silico technologies to test drug efficacy, toxicity and identify potential adverse reactions, the application of animal testing in the drug development process is still a necessity Current technology used to mimic the complexity of the human body in a closed system is still not representative enough to completely render animal testing unnecessary.
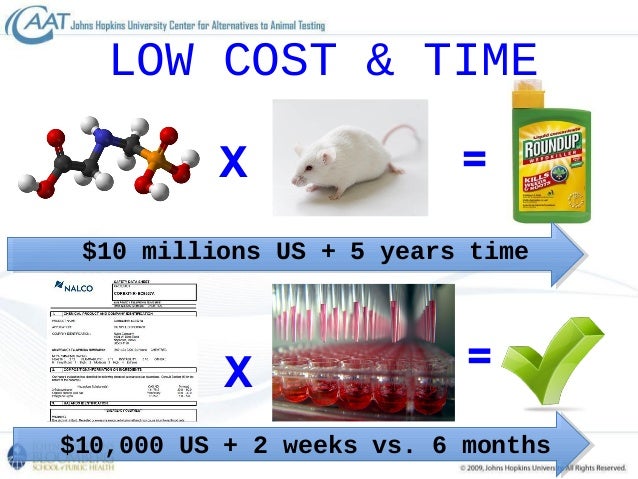
The use of concentrations relative to. In vitro test $,000 Sister chromatid exchange animal test $22,000 in vitro test $8,000 Unscheduled DNA synthesis animal test $32,000 in vitro test $11,000 Eye irritation/corrosion Draize rabbit eye test animal test $1,800 Bovine corneal opacity and permeability (BCOP) test in vitro test $1,400 Skin corrosion. The in silico method can be interpreted as testing that involves a computer or use of computer simulation in experimental research Common ways that in silico methods are incorporated into experiments are Planning experiments and power analysis computer tools are used to improve the design of in vivo and in vitro experiments.
Humane Society International found that animal tests were more expensive than in vitro (testing performed outside of living organisms) in every scenario studied 61 Read More. Yes No 2) Does a product being tested on. Cells in culture are easier to molecularly manipulate, faster, cheaper and more reproducible than animal models Importantly, human cells can be studied in vitro and offer the potential of reducing animal use in several areas of study Many different kinds of cells are available to use in research, including established cell lines and stem cells Because stem cells have the ability to differentiate into many different types of cells, researchers are excited about their use as research models.
Vitro test systems for toxicity testing (Liebsch et al, 11;. In 1980, The New York Times featured a fullpage ad from an animal rights group, which lambasted a prominent cosmetics company for testing its products on the eyes of rabbitsThe campaign was so. The use of concentrations relative to.
Alternatives to animal testing are the development and implementation of test methods that avoid the use of live animals There is widespread agreement that a reduction in the number of animals used and the refinement of testing to reduce suffering should be important goals for the industries involved Two major alternatives to in vivo animal testing are in vitro cell culture techniques and. Cells in culture are easier to molecularly manipulate, faster, cheaper and more reproducible than animal models Importantly, human cells can be studied in vitro and offer the potential of reducing animal use in several areas of study Many different kinds of cells are available to use in research, including established cell lines and stem cells Because stem cells have the ability to differentiate into many different types of cells, researchers are excited about their use as research models. Indeed more than 70% of all in vitro assays captured by the survey were used since 10, peaking at just over 190,000 in 12 To be clear, reducing animal usage is not as simple as swapping out an animal assay for an in vitro alternative Alternatives can’t always tell us, for instance, if a compound will cause DNA damage leading to cancer, but they can some of the time, so we may be able to avoid using animals in the case of compounds with well understood adverse pathways.
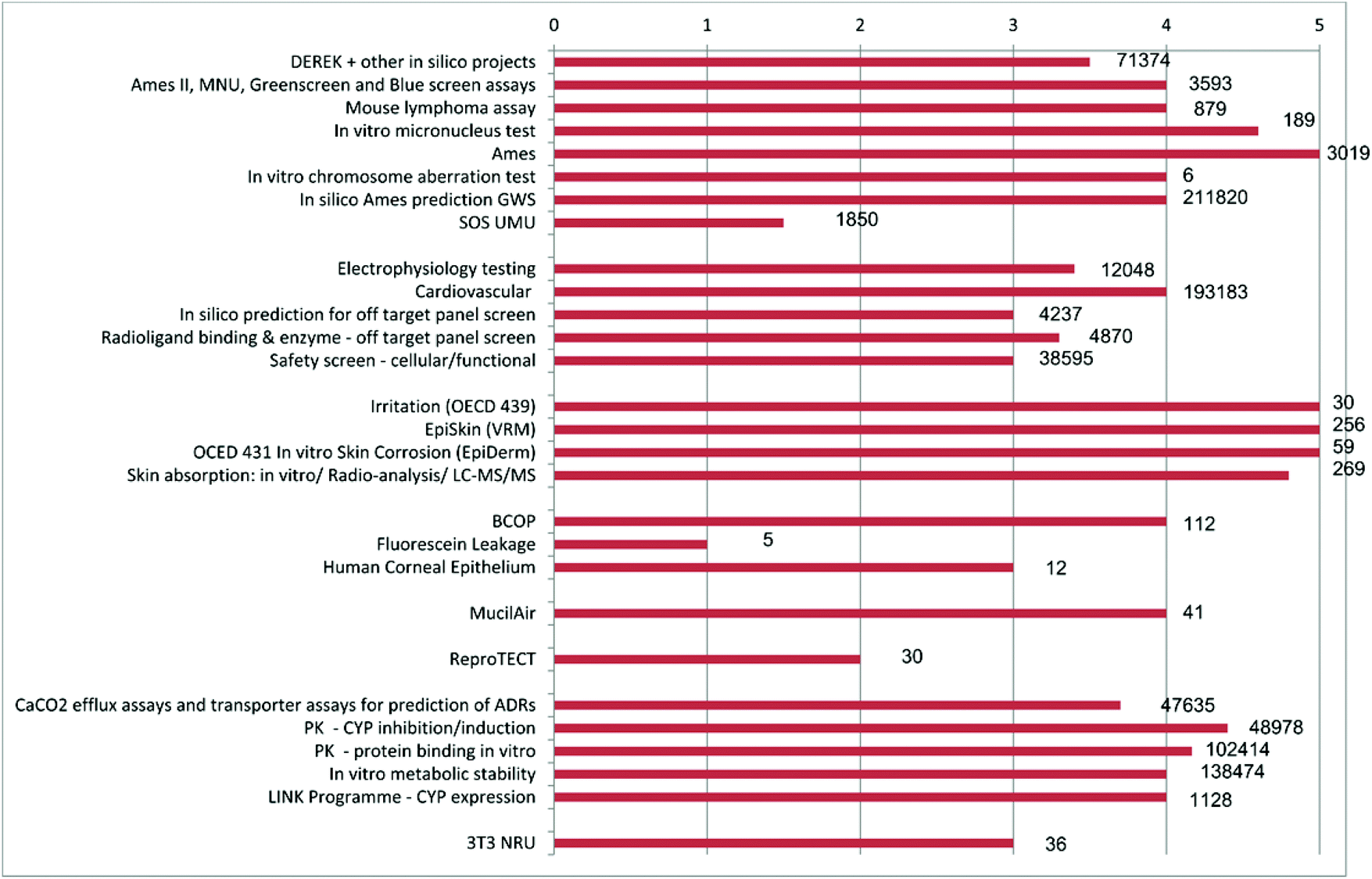
Side a living organism and used for testing Although animals are still required as a source for these in vitro systems, the animal would experience distress for a much shorter time, and perhaps less distress overall, than occurs with wholeanimal testing because it would be killed before any experimental manipulations were carried out. Development and use of in vitro alternatives to animal testing by the pharmaceutical industry 1980–13 JenYin Goh,a Richard J Weaver,b Libby Dixon,a Nicola J Platta and Ruth A Roberts*†c We examined the use of in vitro (including in silico) techniques in preclinical safety testing by the pharma. Animal testing has, to date, of a wider move towards the integration of computer models for drug safety testing which includes the Comprehensive in vitro Proarrhythmia Assay initiative,.
In vitro testing occurs in a laboratory and usually involves studying microorganisms or human or animal cells in culture This methodology allows scientists to evaluate various biological. An in vitro test for detecting eye irritants, the BCOP test method uses tissues obtained from slaughterhouses to replace the use of live animals Federal agencies have accepted ICCVAM’s recommendation to use this test for identification of products that may cause severe or permanent eye damage. In vitro assays bring a number of technical advantages to the conventional method of testing substances on animal models, as outlined, for example, in the 07 US National Research Council (NRC) report These advantages include the ability to elucidate cellularresponse networks and toxicity pathways;.
Development and use of in vitro alternatives to animal testing by the pharmaceutical industry 1980–13 JenYin Goh a, Richard J Weaver b, Libby Dixon a, Nicola J Platt a and Ruth A Roberts† * c a The Association of the British Pharmaceutical Industry (ABPI), 7th Floor, Southside, 105 Victoria Street, London, SW1E 6QT, UK b Institut de Recherches Internationales Servier (IRIS), rue. A replacement alternative is a test method that has been endorsed by an appropriate authority as being capable of fully replacing an existing regulatory test method with a method that does not use live animals in vitro (primary cultures, cell lines, 3D cell culture), exvivo (isolated animal tissues and organs) or in silico (mathematical. Two major alternatives to in vivo animal testing are in vitro cell culture techniques and in silico computer simulation However, some claim they are not true alternatives because simulations use data from prior animal experiments and cell cultures often require animal derived products, such as serum or cells.
Determining drug safety and efficacy requires testing in animals Microfluidic chip testing Microfluidic chips contain tissue samples from different parts of the body that are linked by microchannels through which a blood substitute flows, mimicking pathways and processes in the body This testing method provides more complex information than in vitro tests Studying biological and disease. New Jersey Senator, Cory Booker, is one with a great animal rights track record Where cosmetic testing is concerned, he championed reforms to the Toxic Substances Control Act in 16 In addition to this, Booker has made a full ban on animal testing part of his presidential platform Former Secretary of Housing and Urban Development Julian Castro. Scientists often study the effects of drugs and chemicals on animals before they deem them safe for humans When possible, they try to perform these toxicology tests using biochemical or cellbased (in vitro) systems instead of with animals such as mice.
These alternatives to animal testing include sophisticated tests using human cells and tissues (also known as in vitro methods), advanced computermodeling techniques (often referred to as in silico models), and studies with human volunteers These and other nonanimal methods are not hindered by species differences that make applying animal test results to humans difficult or impossible, and they usually take less time and money to complete. Invivo testing studies living tissues within the organism, and invitro testing studies the living tissue outside the body To study the effect of the same pathogen on living tissue with invivo testing, the pathogen would be injected into a living organism, like an animal, and the entire animal would be studied as the pathogen acts out its course. In Vitro testing is typically the first type of tests that are run on any ingredient that will go into a cosmetic In Vivo testing – This refers to experiments on humans or small animals to determine the effect of an ingredient or formula.
Prior to animal testing and clinical trials, traditionally, when testing pharmaceutical ingredients, the first step has been to test in vitro using 2D cell cultures 7 This has however provided limitations with respect to testing as the results obtained from in vitro 2D cell culture studies have not translated to in vivo studies 7. Preclinical in vitro tests, preclinical animal tests, Phase I clinical trials, and Phase IIIII clinical trials all work successively to remove potentially dangerous compounds from reaching the market These are not their only functions, animal tests may help assess appropriate therapeutic doses, which can be later refined during clinical trials. Animal testing Using animals in tests and experiments to see the outcome In vitro testing Test tube experimenting Survey.
Considering the ethical issues and the cost of invivo animal tests, the pharmaceutical industry now relies more on invitro methods for toxicity testing in the drug development phase Here, we answer the common questions regarding the invitro toxicity testing in drug development. Krug et al, 13) A A letter published in the current issue of this jour nal discussed the impressive progress made in. In vitro testing is going to protect animals and it is actually more effective because animal responses will differ from a human response The organsonchips use actual human cells, therefore the cell response is way more efficient than testing on animals.
PETA advocates instead for moremodern alternatives such as in vitro testing using human cells and computer modeling On the other hand, others would argue that conducting experiments and research. Given the key characteristics of in vitro tests (costs, throughput, animal use) they should play a role in an early phase of a testing strategy, not as the mechanistic tests at the end to further explain in vivo findings. This will decrease the use of animals in the testing process Furthermore, SC can show a specific organ’s reaction to a substance Nevertheless, in vitro techniques won’t show how the entire human system would respond Therefore, investigations with animals are still needed because animal models are being considered the most similar model to represent the human body Hence, these in vivo experiments will increase our knowledge and contribute to a better human but also a better animal.
Development and use of in vitro alternatives to animal testing by the pharmaceutical industry 1980–13 JenYin Goh a, Richard J Weaver b, Libby Dixon a, Nicola J Platt a and Ruth A Roberts† * c a The Association of the British Pharmaceutical Industry (ABPI), 7th Floor, Southside, 105 Victoria Street, London, SW1E 6QT, UK b Institut de Recherches Internationales Servier (IRIS), rue. Animal Testing vs in vitro testing Animal testing Using animals in tests and experiments to see the outcome In vitro testing Test tube experimenting Survey 1) Are you aware if your beauty/hygiene products are animal tested or not?. In vivo (Latin for “within the living”) refers to experimentation using a whole, living organism as opposed to a partial or dead organism Animal studies and clinical trials are two forms of in vivo researchIn vivo testing is often employed over in vitro because it is better suited for observing the overall effects of an experiment on a living subject.
In vitro techniques focus on the cellular level and therefore cannot replace wholebody testing;. Side a living organism and used for testing Although animals are still required as a source for these in vitro systems, the animal would experience distress for a much shorter time, and perhaps less distress overall, than occurs with wholeanimal testing because it would be killed before any experimental manipulations were carried out. Preclinical in vitro tests, preclinical animal tests, Phase I clinical trials, and Phase IIIII clinical trials all work successively to remove potentially dangerous compounds from reaching the market These are not their only functions, animal tests may help assess appropriate therapeutic doses, which can be later refined during clinical trials.
PIP Some evidence exists that invitro studies are capable or potentially capable of providing more rapid, precise, and relevant information than do some animal studies At this time, the primary use of invitro tests is the detection of specific toxic properties of drugs and chemicals, eg, mutagenesis and mechanisms of toxicity. Since the ban on animal testing in Europe, and its extension to many parts of the world, a battery of in vitro tests covering the key steps of the Adverse Outcome Pathway (AOP) for skin sensitization is recommended To date, three in vitro methods are validated in the OECD guidelines (442C, 442D, 442E), and many others are under validation by. The fact is that such methods currently tend to complement, rather than replace, the use of.
A Animal test = $1800 per assay b In vitro alternative 1 (EpiDerm) = $850 per assay In vitro alternative 2 (Corrositex) = $725 per assay 5 Genotoxicity (if the chemical is toxic to DNA) a Animal test of chromosomal aberration = $30,000 per assay In vitro alternative test = $,000 per assay b Animal test of sister chromatid exchange = $22,000 per assay. In vitro methods integrated with computational models can replace animal tests Toward new alternatives to animal testing (21, January 14). In Vitro testing is typically the first type of tests that are run on any ingredient that will go into a cosmetic In Vivo testing – This refers to experiments on humans or small animals to determine the effect of an ingredient or formula.
The 3T3 Neutral Red Uptake Phototoxicity Test can replace the use of mice and other animals in the testing of medicines and other products for their potential to cause sunlight induced "phototoxicity" A combination of in vitro test methods and already available human and animal data may be used to determine the potential for skin sensitization. Animal test $32,000 in vitro test $11,000 Eye irritation/corrosion Draize rabbit eye test animal test $1,800 Bovine corneal opacity and permeability (BCOP) test in vitro test $1,400 Skin corrosion Draize rabbit skin test animal test $1,800 EpiDerm™ human skin model in vitro test $850 CORROSITEX® membrane barrier in vitro test $500 Skin sensitisation. One of the biggest hurdles to replace in vivo animal studies with in vitro humanrelevant tests is a lack of correlation between the results of human and animal studies Toxicologists know that animal studies are not always reproducible and useful for predicting adverse human health effects.

Uk Government Bans Animal Testing On Household Products Cyprotex

Can Animal Research Be Applied To Humans Speaking Of Research

Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd
In Vitro Testing Vs Animal Testing のギャラリー

3rs Of Animal Testing For Regenerative Medicine Products Science Translational Medicine
Ec Europa Eu Transparency Regexpert Index Cfm Do Groupdetail Groupdetaildoc Id No 7
Www Mdpi Com 79 9284 4 3 30 Pdf

How Omics Technologies Can Contribute To The 3r Principles By Introducing New Strategies In Animal Testing Trends In Biotechnology

Reducing Animal Testing Using Stem Cells
Alternative Non Animal Methods For Cosmetics Testing Current Status And Future Prospects 10 Springerlink

The Ascendance Of Microphysiological Systems To Solve The Drug Testing Dilemma Future Science Oa

Animal Testing In Eu Declining Cosmetics Must Share In Vitro Methods

Animal Research Statistics For Germany In 13 Speaking Of Research
Animal Testing Science Or Tradition
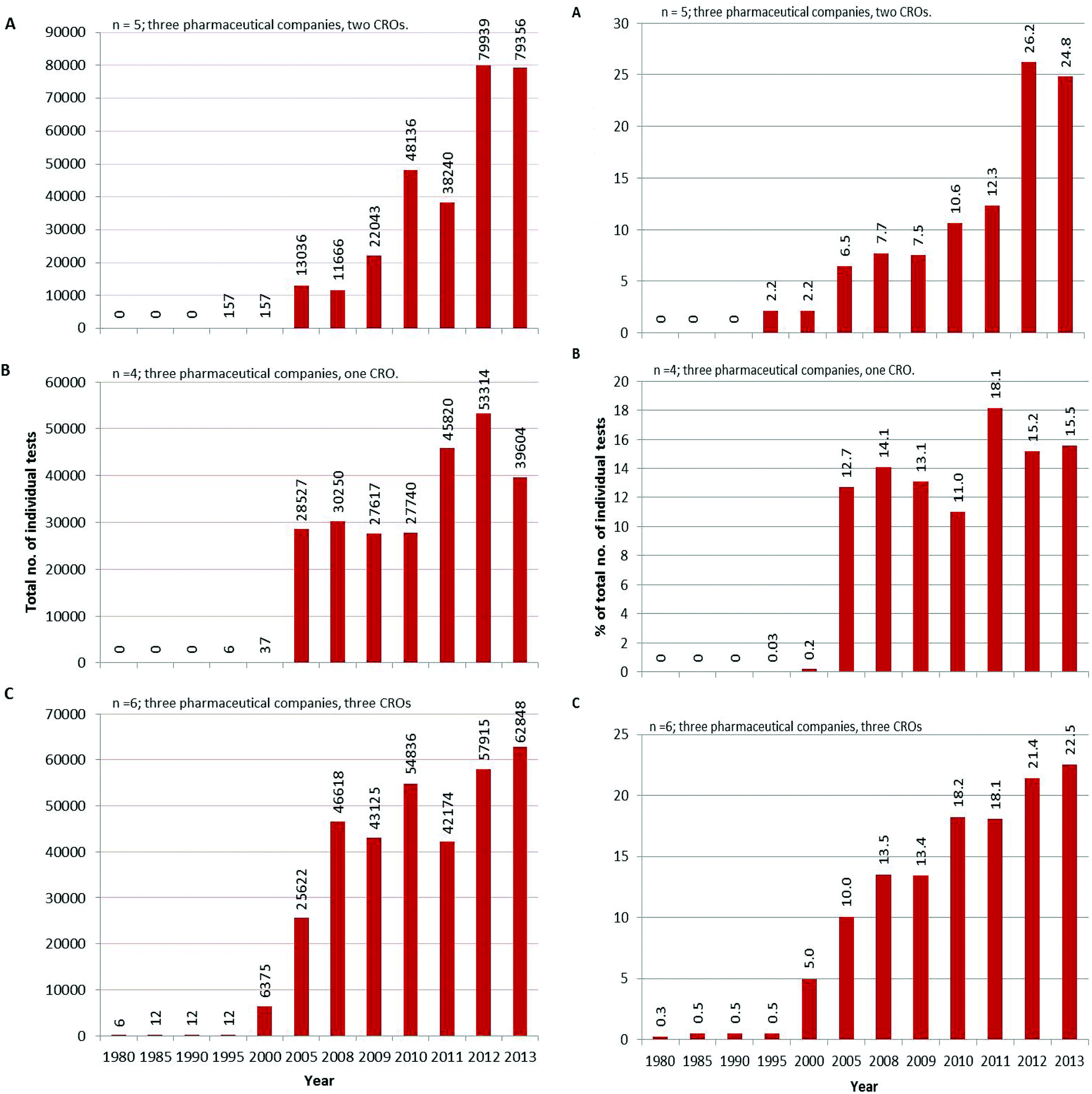
Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd

Echa Animal Testing Alternatives Report For Reach Regulation

Perfectus Biomed Great Talk On Emerging And Approved In Vitro Alternative Methods To Animal Testing Episkin

In Vitro The Remarkable Rise Of Animal Alternatives Understanding Animal Research Understanding Animal Research

Thesis For Animal Testing Pro

A Tiered Approach To The Use Of Alternatives To Animal Testing For The Safety Assessment Of Cosmetics Eye Irritation Sciencedirect
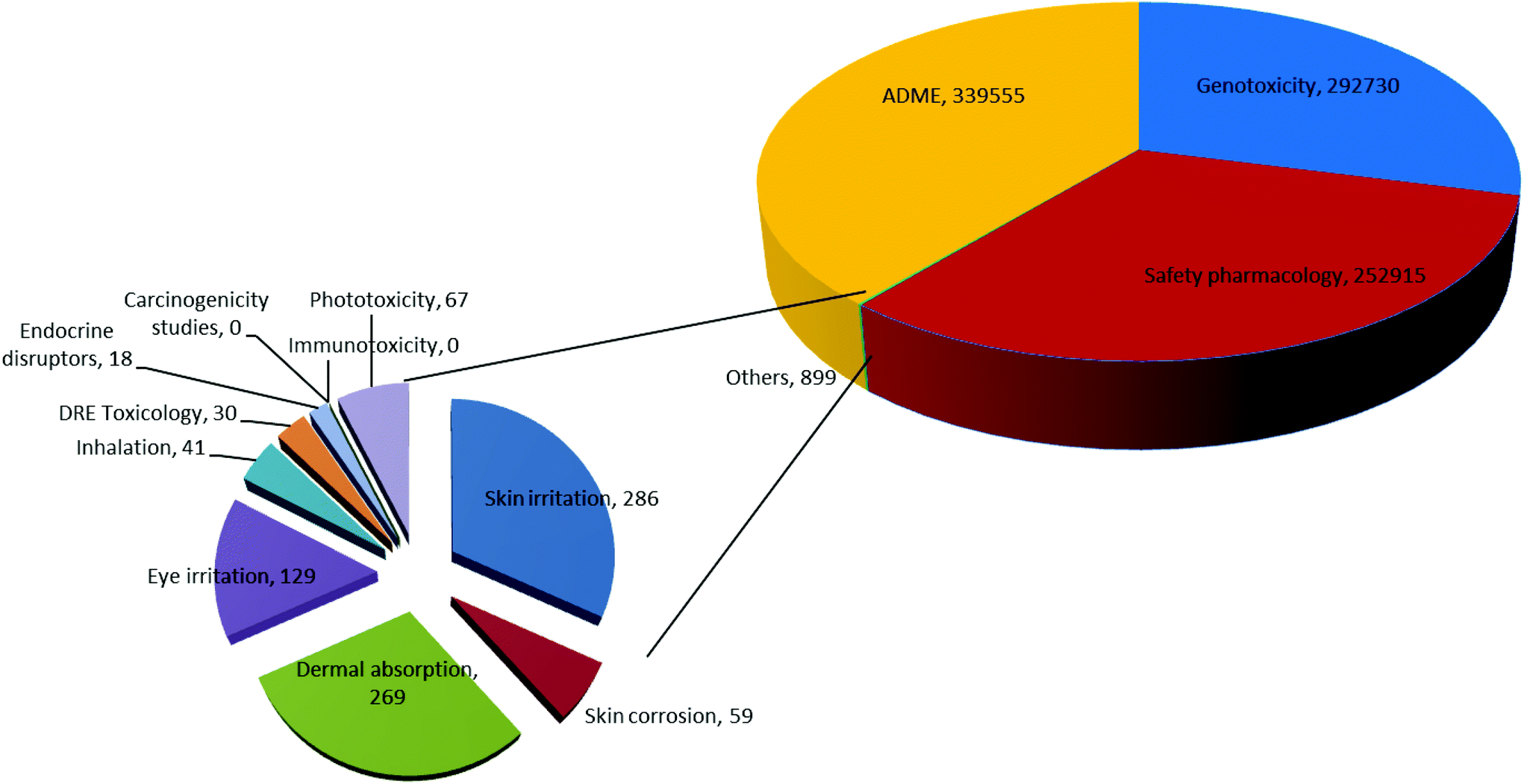
Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd
Q Tbn And9gcqdd7vuq6bglybo9hvyrhiens12zuyc6c7up2qzzdjip4xfcycu Usqp Cau

Animal Testing Labs In India Cognibrainmanuscript

Singapore Scientists Create In Vitro Human Skin As Alternative To Animal Testing Global Cosmetics News

Alternatives To Animal Testing Wikipedia
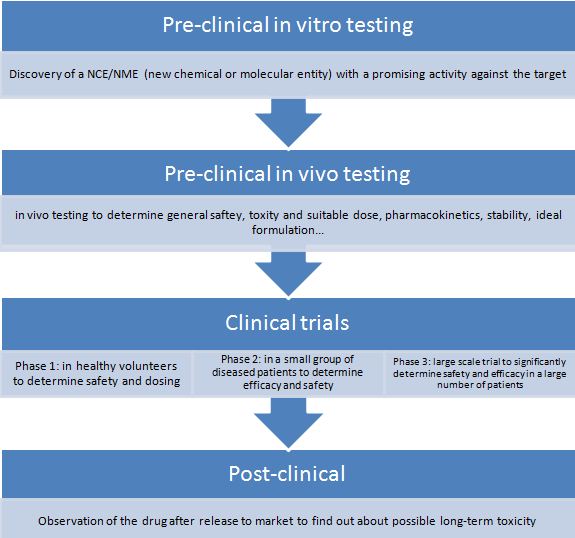
Animal Testing The Bridge Between English2biotech

Predicting Acute Fish Toxicity Using In Vitro Models Eawag

Animal Cruelty For Cosmetics Visual Ly

Figure 3 From Animal Testing And Its Alternatives The Most Important Omics Is Economics Semantic Scholar

Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd
Are In Vitro And In Silico Approaches Used Appropriately For Animal Based Major Depressive Disorder Research

Alternatives To Animal Testing

The Use Of Various Approaches To Phase Out Animal Testing Download Scientific Diagram

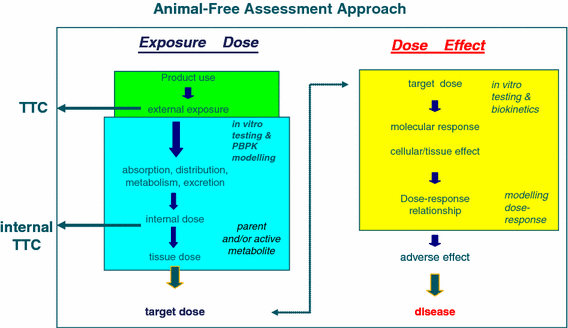
The Drug Testing Dilemma The Graph Illustrates Schematically The Route Download Scientific Diagram
Q Tbn And9gctgibmexkgkzkza1nj3anqutm1zdag2xcoibq C5vkrsegnsflc Usqp Cau

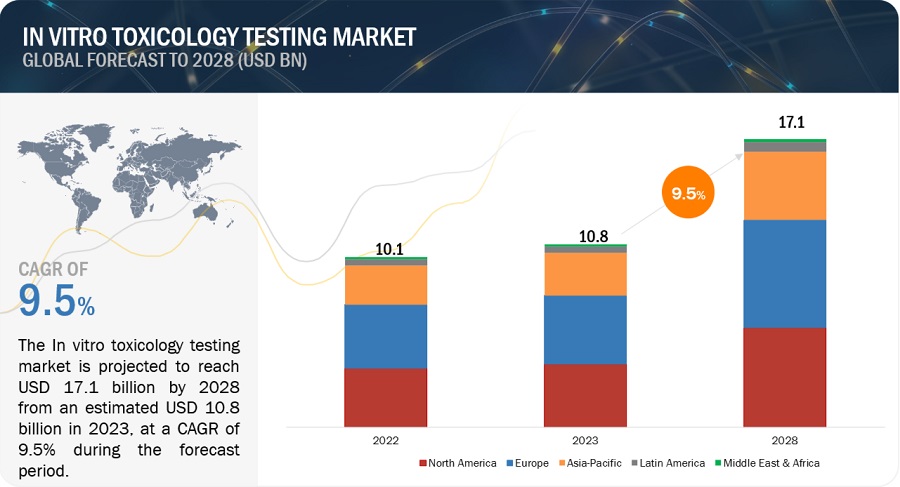
In Vitro Toxicity Testing Market Global Forecast To 25 Marketsandmarkets

Principles For In Vitro Toxicology Sciencedirect

Alternatives To Animal Testing
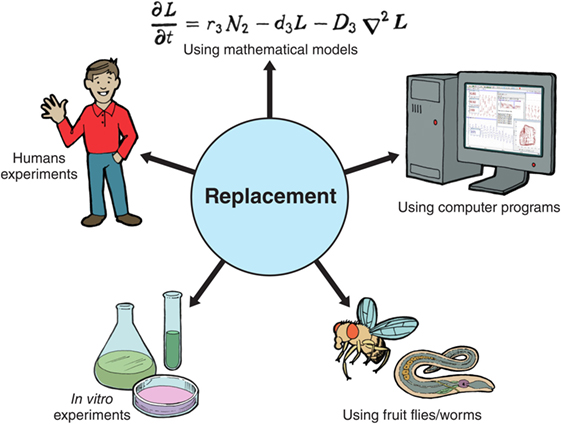
The 3rs What Are Medical Scientists Doing About Animal Testing Frontiers For Young Minds

Xcellr8 Linkedin

Alternatives To Animal Screening Methods P Screening Mohammadhusain

In Vivo Vs In Vitro Drug Development Youtube

Pdf Animal Testing And Its Alternatives The Most Important Omics Is Economics
Q Tbn And9gcsrgsuwfvgqlsfuhdlxphkrz37ggop6 Qpywiimpuz6fw1notlz Usqp Cau

Advantages And Disadvantages Of Biocompatibility Test Methods 2 Download Table

Pharmaceutical Toxicology Designing Studies To Reduce Animal Use While Maximizing Human Translation Sciencedirect

Animal Testing Brochure Animal Testing Toxicity
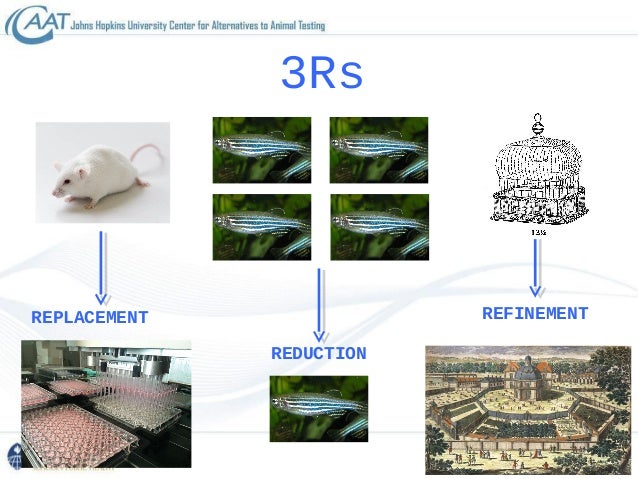
Introduction To Alternatives To Animal Testing In Toxicology

The Alternatives To Animal Testing Animals Are Not Ours To Experiment On Peta Uk
In Vitro Toxicity Testing Market Global Forecast To 25 Marketsandmarkets
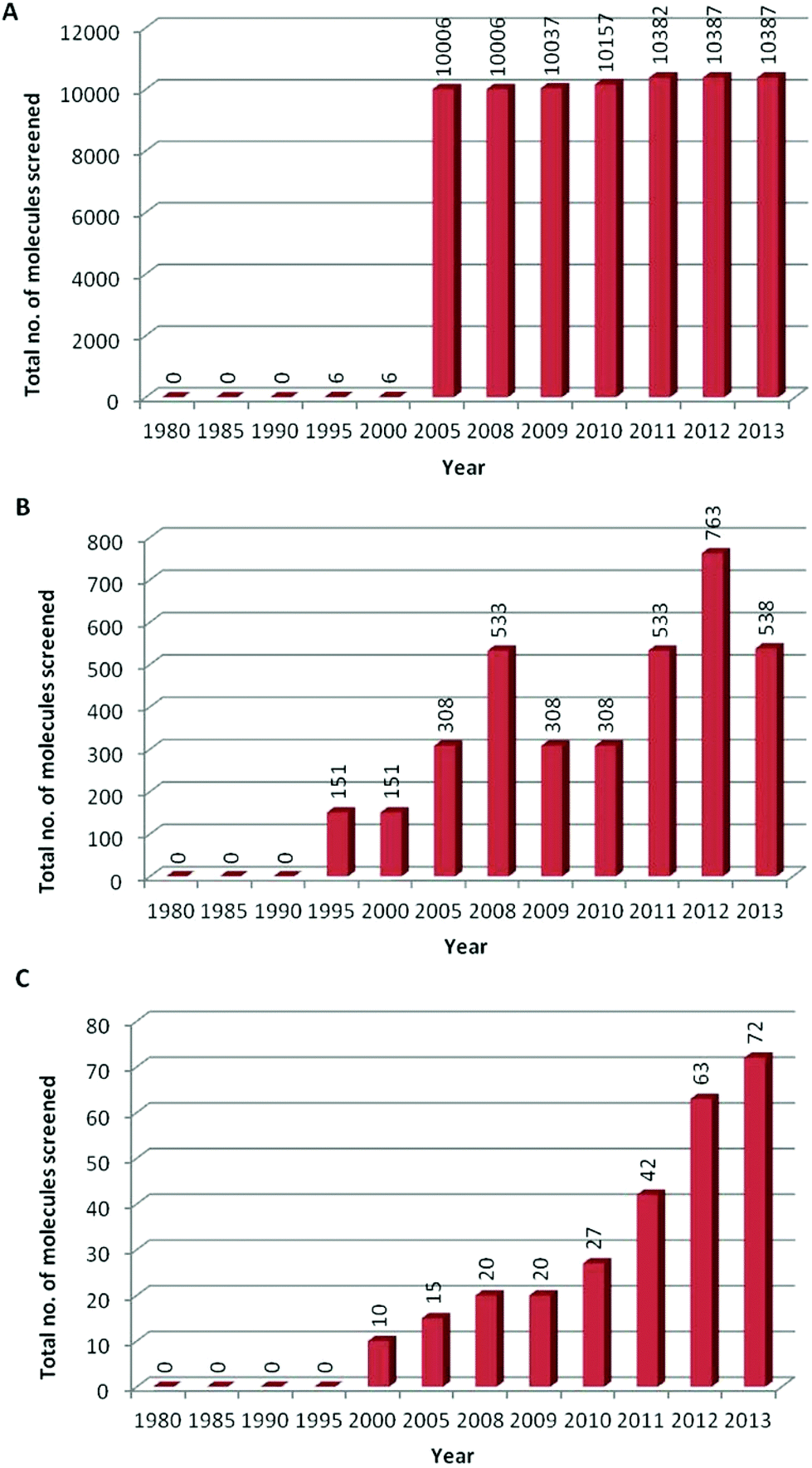
Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd

What Is Animal Testing Facts Pros Cons Alternatives

In Vitro Toxicology Toxicity Testing Market Global Opportunity Analysis And Industry Forecast 19 25 Meticulous Market Research Pvt Ltd

In Vitro Non Animal Testing Using The Reconstructed Human Epidermis Model Cyprotex

Animal Experiments Testing Times Science Technology The Economist
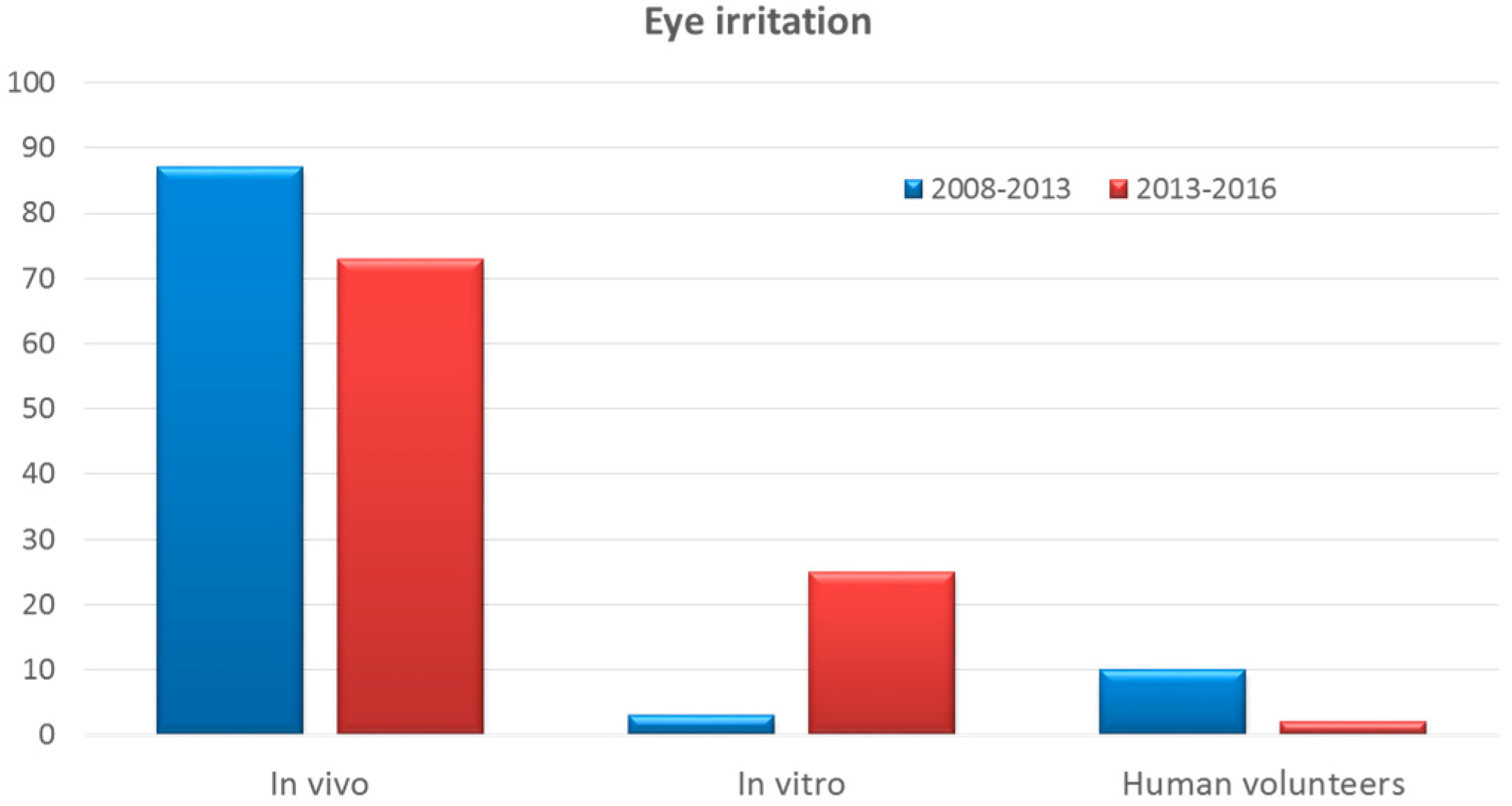
Cosmetics Free Full Text Alternative Methods To Animal Testing For The Safety Evaluation Of Cosmetic Ingredients An Overview

Alternates To Animal Testing Dabur Research Foundation
The Alternatives To Animal Testing Animals Are Not Ours To Experiment On Peta Uk

Number Of Animal Experiments In Uk Rose In 12 Speaking Of Research
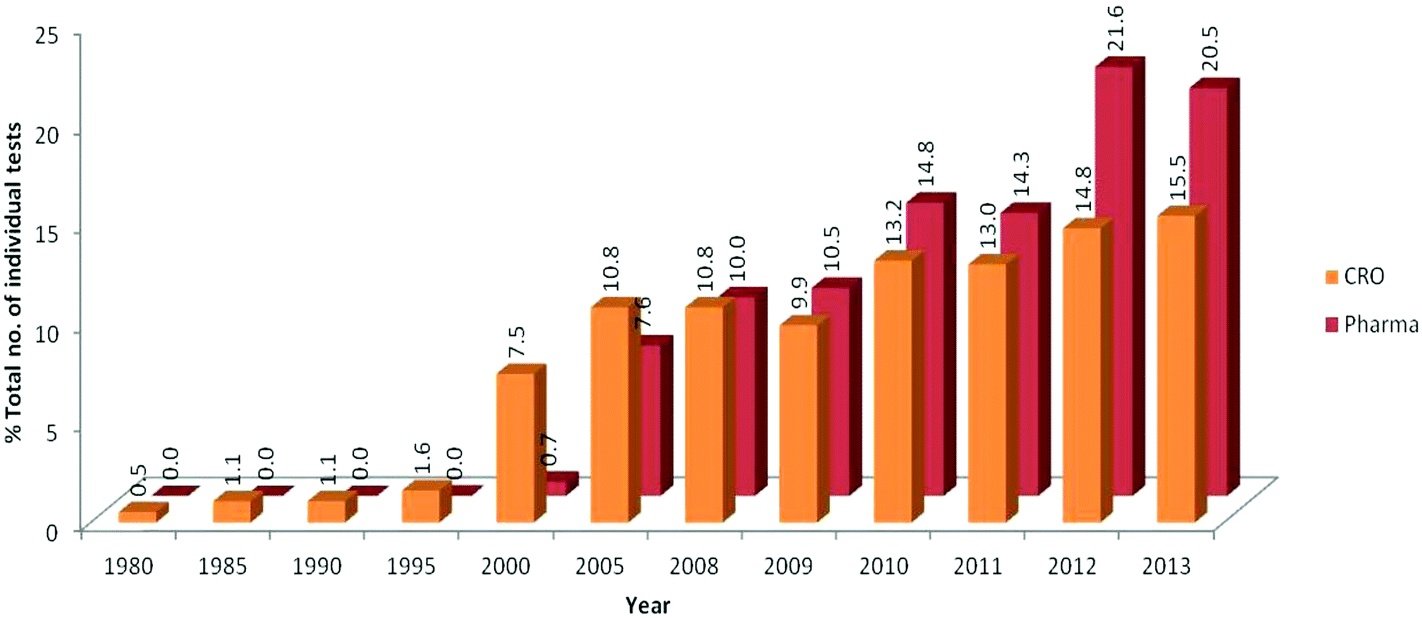
Development And Use Of In Vitro Alternatives To Animal Testing By The Pharmaceutical Industry 1980 13 Toxicology Research Rsc Publishing Doi 10 1039 C5txd

Alternative Methods To Animal Testing Review

Reach Taking Up Alternatives To Animal Testing Kosmetica World

Examples Of In Vitro And In Vivo Methods Specific Biological Download Scientific Diagram

Saving The Animals New Ways To Test Products The New York Times

In Vitro Genotoxicity Testing Can The Performance Be Enhanced Sciencedirect

Preclinical Animal Testing Requirements And Considerations Sciencedirect

Alternatives To Animal Testing Cruelty Free International

Animal Welfare And Non Animal Testing For Regulatory Purposes Croner I

General Procedures For Safety Tests On Carbon Nanomaterialsprocedures For Sample Preparation Characterization In Vitro Cell Based Assays And Animal Tests On Carbon Nanomaterials Riss

In Vivo Vs In Vitro Differences Between In Vitro Vs In Vivo 7esl

Animal Tests Ban For Cosmetics Debate On A Ep S Resolution 16 November Palace Of Nations Geneva Oipa

Alternatives To Animal Testing Wikipedia
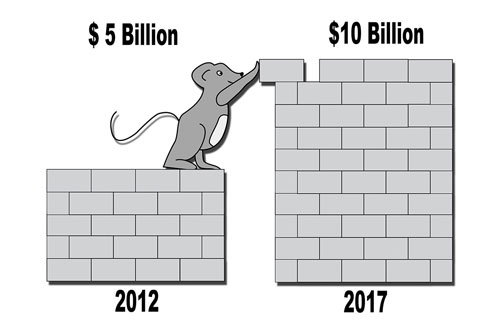
Alternatives To Animal Testing Drive Market

In Vitro Non Animal Testing Using The Reconstructed Human Epidermis Model Cyprotex
In Vitro Methods And More Animal Testing Alternatives Peta
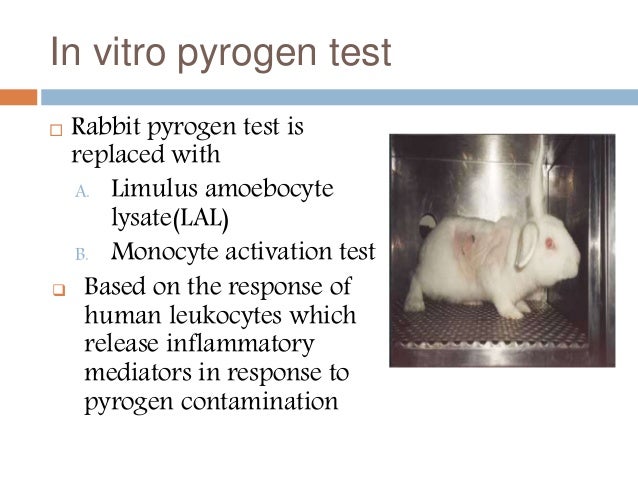
No More Animal Based Pyrogen Tests One In Vitro Test To Detect Endotoxin Non Endotoxins Pyrogens Now Is The Time Anticipate The Health Authority Requirements Shift To In Vitro Testing

Pdf Eurl Ecvam Strategy To Avoid And Reduce Animal Use In Genotoxicity Testing Semantic Scholar
Alternatives To Animal Experiments

The Global In Vitro Toxicology Testing Market Is Estimated To Witness A Cagr Of 8 2 During The Forecast Period 18 24 The Glob Marketing Forecast Global

In Vitro Citizens For Alternative To Animal Research

Echa Newsletter Home

Relevance Of Animal Models For Wound Healing Wounds Research

Alternatives Stop Animal Testing
Cosmetics Free Full Text Alternative Methods To Animal Testing For The Safety Evaluation Of Cosmetic Ingredients An Overview Html
X Cellr8 Com Wp Content Uploads 03 Xcell Hpc3 Pdf

10 Animal Testing Research Ideas Animal Testing Stop Animal Testing Animals

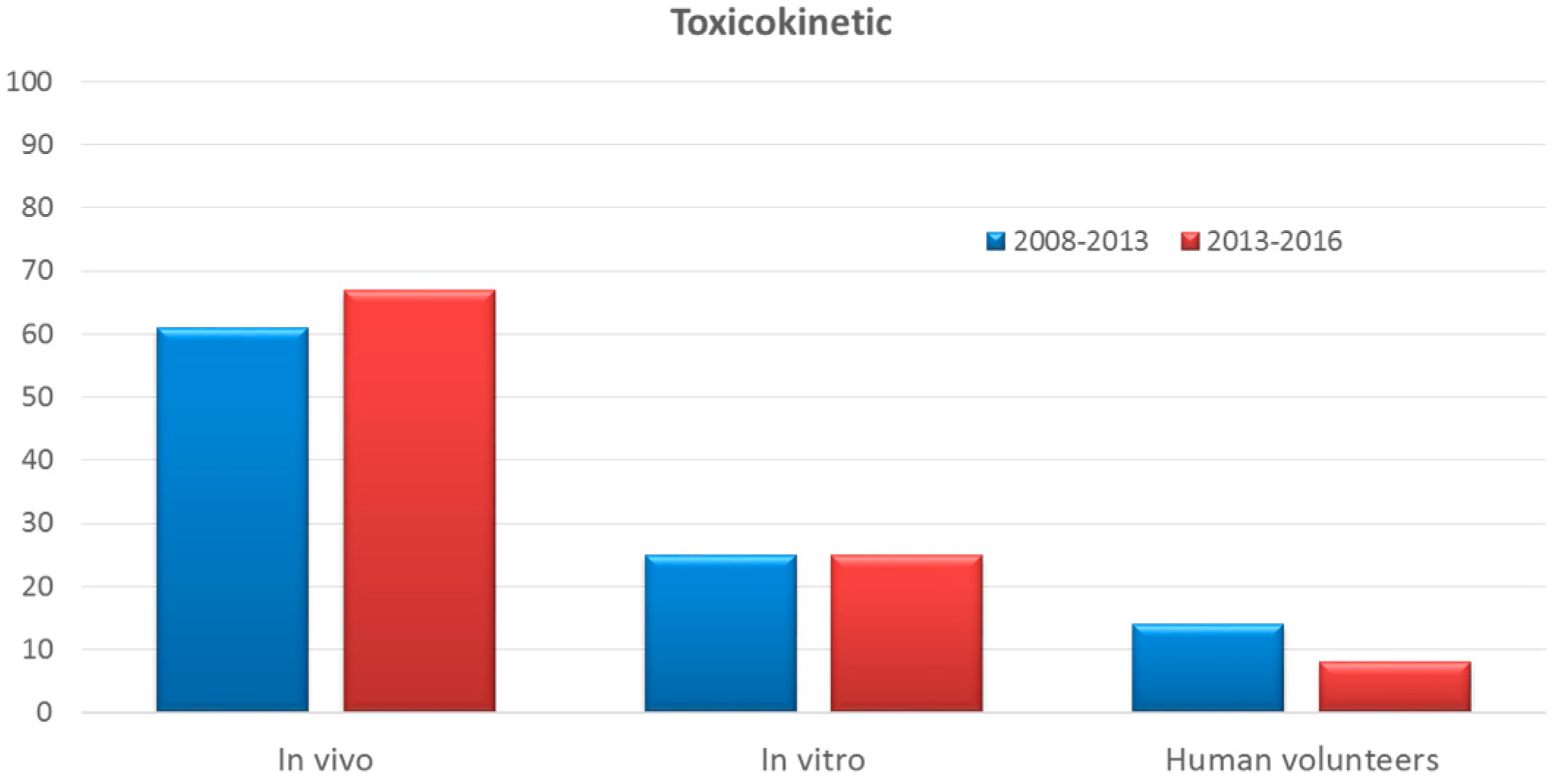
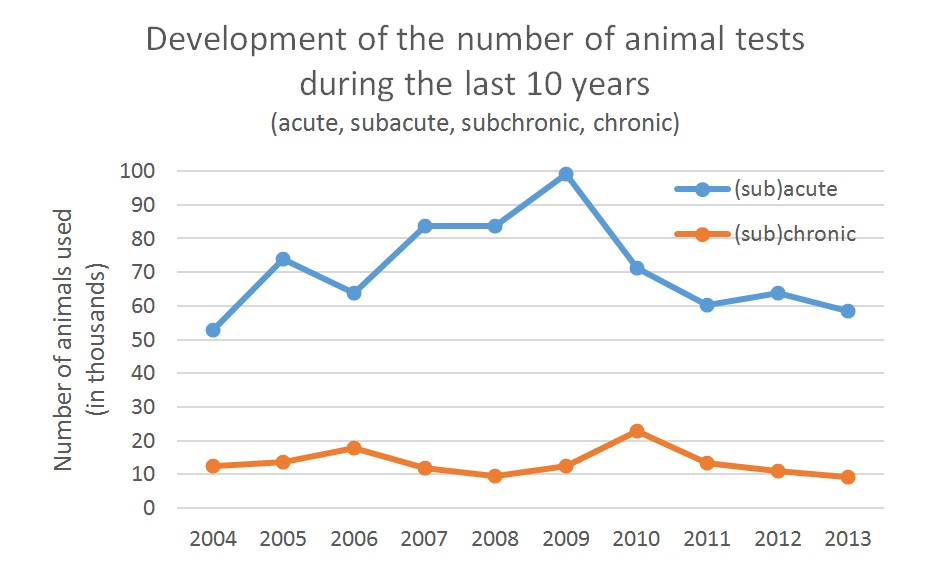
Comparison Of The Number Of In Vivo And In Vitro Tests Performed Per Download Scientific Diagram
Working Group A Portrait Tissuse Gmbh
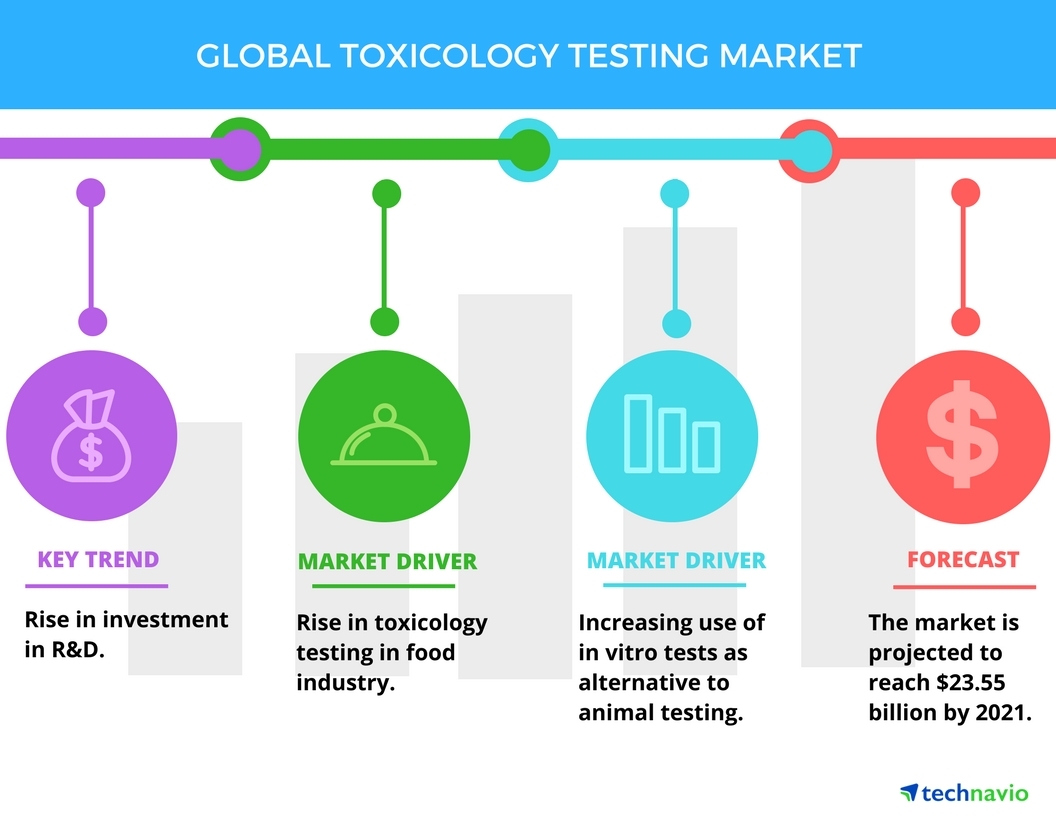
Top 3 Drivers In Toxicology Testing Market Technavio Business Wire

Toxicity Tests Alternative Methods In Toxicology Prof Dimitrios Kouretas Ppt Download
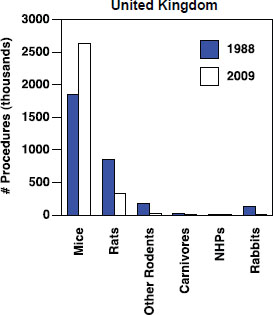
4 Animals In Neuroscience Research International Animal Research Regulations Impact On Neuroscience Research Workshop Summary The National Academies Press

Alternative Methods To Animal Testing Ocular Irritation Assays Youtube
Hsi India Teams Up With Reagene Biosciences To Advance Non Animal Testing Research In India Humane Society International

The Many Benefits Of Using Alternatives To Animal Testing Invitrointl

Announcements Altex Alternatives To Animal Experimentation
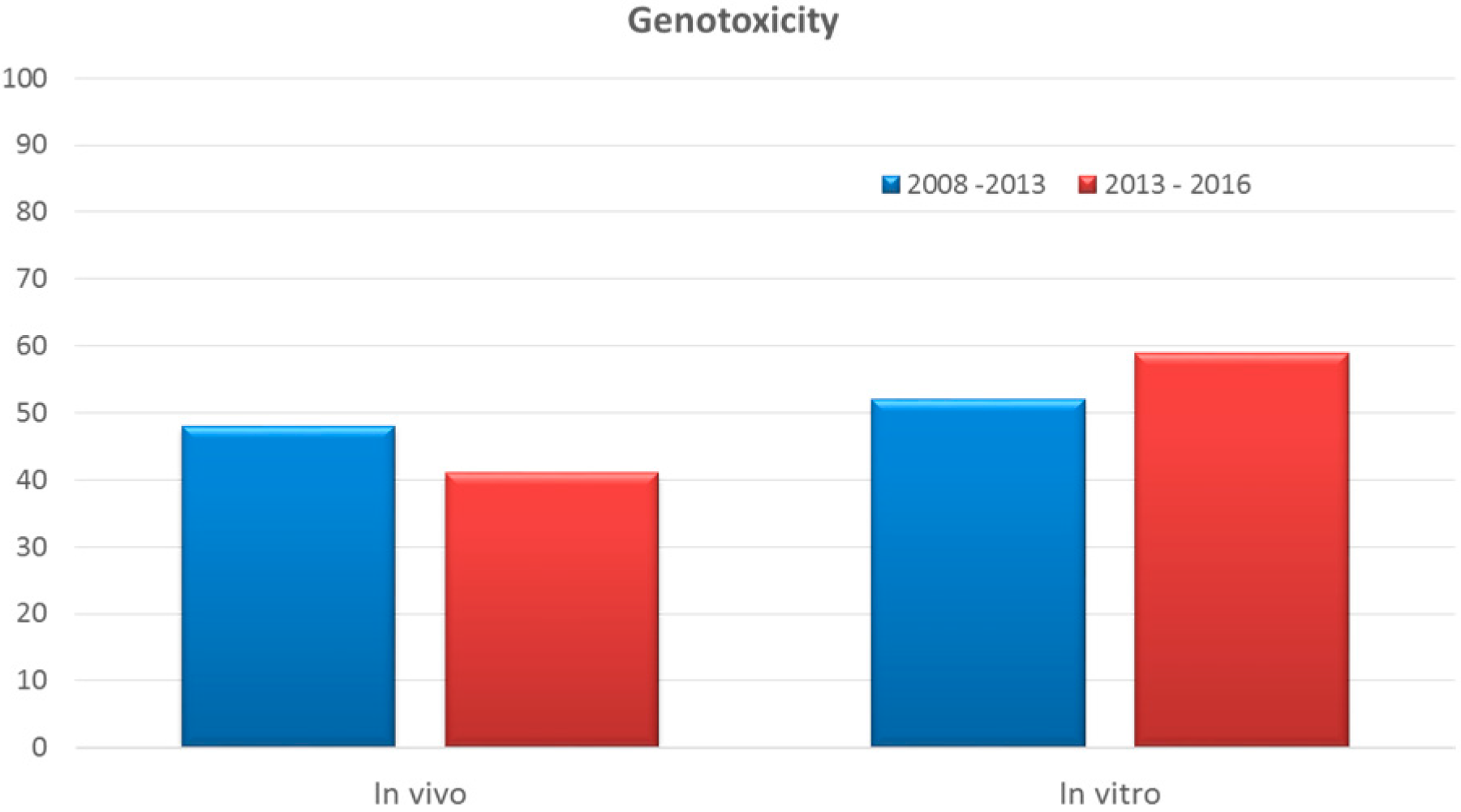
Cosmetics Free Full Text Alternative Methods To Animal Testing For The Safety Evaluation Of Cosmetic Ingredients An Overview
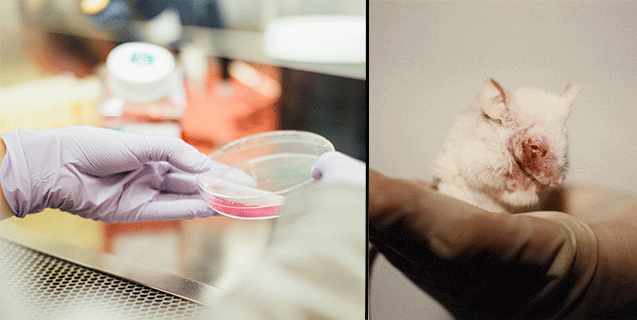
In Vitro Methods And More Animal Testing Alternatives Peta
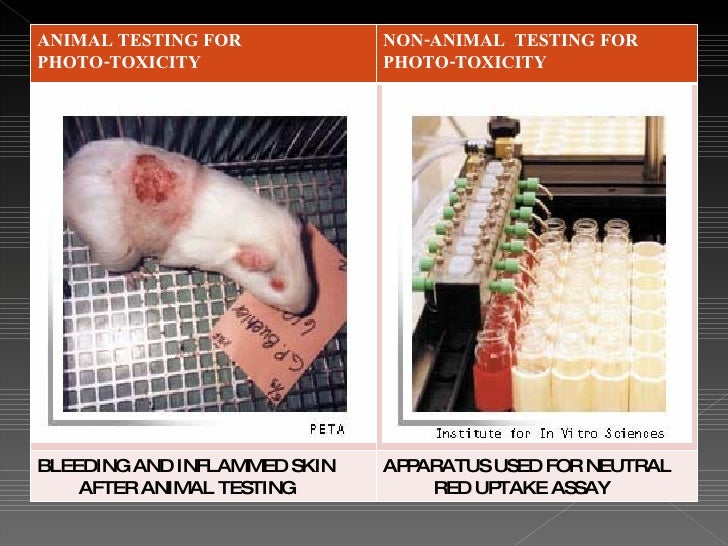
Alternatives To Animal Testing

Selected In Vitro And In Vivo Testing Methods For Short Term Download Table

In Vitro Testing

Regan S Animal Rights Outman Environmental Blog

Efforts To Replace And Reduce The Use Of Animals In Genotoxicity Testing Download Scientific Diagram

1 Advantage And Disadvantage Of In Vivo And In Vitro Test Download Table
3

Fundamentals Research Animals Faunalytics



