Iec 60601
The internationally recognized standard, IEC , was first published in 1977 Since that time, the standard has been updated and restructured several times The most recent update to IEC was published earlier this year and includes several changes and clarifications.

Iec 60601. Abstract IEC contains requirements concerning basic safety and essential performance that are generally applicable to medical electrical equipment For certain types of medical electrical equipment, these requirements are either supplemented or modified by the special requirements of a collateral or particular standard. IEC has become a global benchmark for electrical medical equipment and many companies view compliance with it as defacto requirement for most markets Some of the medical equipments covered are Patient ventilators;. Copyrighted standards are not included in the material provided 1 Day Limited Seminar Does not cover all the standard requirements of the full seminar, but can be customized to meet your needs.
From the perspective of compliance, IEC is considered one of the costliest standards that has ever been published Older versions of the standard were expensive, costing $10,000 to $,000 for relatively lowrisk products to get certified The newer versions doubled and even quadrupled some of those costs. Today we have the pleasure to announce that the cybersecurity technical report IEC TR has been published The not so short title of this report is Medical electrical equipment – Part 45 Guidance and interpretation – Safety related technical security specifications for medical devices. IEC is a series of technical standards for the safety and essential performance of medical electrical equipment, published by the International Electrotechnical Commission First published in 1977 and regularly updated and restructured, as of 11 it consists of a general standard, about 10 collateral standards, and about 80 particular standards.
The IEC standard is a set of documentation that was made to address the risks that are associated with the use of electronic medical equipment It is in the manufacturer’s best interest to ensure that each device they design and release for purchase is compliant with this set of standards. IEC is a complex standard that trips up many medical device developers For my part, I’d encourage manufacturers to work with trusted partners who are familiar and have experience with the standard, and to do so early on. Benefits of applying the standard and how to demonstrate compliance by downloading Making Green Profitable Using IEC as a Competitive.
Today we have the pleasure to announce that the cybersecurity technical report IEC TR has been published The not so short title of this report is Medical electrical equipment – Part 45 Guidance and interpretation – Safety related technical security specifications for medical devices. IEC is a complex standard that trips up many medical device developers For my part, I’d encourage manufacturers to work with trusted partners who are familiar and have experience with the standard, and to do so early on. IEC applies to the basic safety and essential performance of Medical Equipment (ME) equipment and ME systems in the presence of electromagnetic disturbances and to electromagnetic disturbances emitted by me equipment and me systems This collateral standard to IEC specifies general requirements and tests for basic safety and essential performance with regard to electromagnetic disturbances and for electromagnetic emissions of ME equipment and ME systems.
IEC A112 contains requirements concerning basic safety and essential performance that are generally applicable to medical electrical equipment For certain types of medical electrical equipment, these requirements are either supplemented or modified by the special requirements of a collateral or particular standard. IEC contains requirements concerning basic safety and essential performance that are generally applicable to medical electrical equipment For certain types of medical electrical equipment, these requirements are either supplemented or modified by the special requirements of a collateral or particular standard. Full Description IEC specifies a process for a manufacturer to analyse, specify, design, verify and validate usability, as it relates to basic safety and essential performance of medical electrical equipment.
The Amendments Project under SC62A covers the general standard (IEC ) and most of the collateral standards (IEC XX, except for IEC ) (For background on the Amendments Project, refer to my previous article, “The Future of the IEC Series An Update,” published in the In Compliance Annual Reference Guide). IEC/EN has wording that addresses the use of radios in a medical device An exemption for the main transmit signal from the radiated emissions limits (provided that they meet the national requirements) is given, but all other emissions must meet the radiated emissions limits of IEC/EN. I EC “Medical electrical equipment,” edition 31, is the base medicaldevice standard to ensure “basic safety and essential performance” of medical electrical equipment.
The standard family IEC is actually only applicable to medical electrical devices But IEC is an exception This standard for IT security has all medical products in the scope that they are integrated into IT networks This also affects software as a medical device. From the perspective of compliance, IEC is considered one of the costliest standards that has ever been published Older versions of the standard were expensive, costing $10,000 to $,000 for relatively lowrisk products to get certified The newer versions doubled and even quadrupled some of those costs. IEC is a series of technical standards that ensure the safety of medical electrical equipment IEC (Edition 32) deals with the basic safety and essential performance requirements of medical electrical equipment, and serves to ensure that no single electrical, mechanical, thermal or functional failure shall pose an unacceptable risk to patients and/or operators.
IEC is the primary standard governing the design of medical devices While not all countries have adopted IEC as the standard, globally it has become the de facto international benchmark for the design of electronic medical devices. MECA Ed 31 Evaluation Package (BETA) The Evaluation Package is a summary of the IEC standard, other applicable requirements, guidance information, and interpretations, to help evaluate medical electrical equipment to the requirements of the Standard. IEC with National Differences (US, AAMI ES , Canada, CSA C222 No , Europe, EN ) Customized to Client’s equipment and needs;.
IEC is a widely accepted series of international standards for the basic safety and essential performance of medical electrical equipment Your new and existing medical devices must demonstrate compliance with the latest revision of IEC We are a Nationally Recognized Testing Laboratory (NRTL) approved by OHSA, providing testing, certification, and inlab support to help you navigate the new requirements of the 3 rd Edition of IEC and to support your safety claims. IEC is a complex standard that trips up many medical device developers For my part, I’d encourage manufacturers to work with trusted partners who are familiar and have experience with the standard, and to do so early on. IEC The International Electrotechnical Commission (IEC) is a worldwide body that promotes international standardization in electronics In 1993 it released the standard, "Medical Electrical Equipment—Part 1 General Requirements for Safety, Amendment No 2.
IEC Clause Requirement Test Result Remark Verdict TRF No IEC_1H 4 GENERAL REQUIREMENTS P 41 Requirements of this standard applied in NORMAL USE and reasonably foreseeable misuse P 42 RISK MANAGEMENT PROCESS FOR ME EQUIPMENT OR ME SYSTEMS P 422 General requirement for RISK MANAGEMENT. The main IEC standard (referred to in Europe as EN and in Canada as CSA ) is an umbrella for numerous subsidiary standards, variously known as “collateral” or “particular” standards. ADVERTISEMENT IEC is considered one of the most costly standards to comply with that has ever been published Older versions of the standard were expensive, costing $10,000 to $,000 for relatively lowrisk products to get certified The newer versions doubled and even quadrupled some of those costs.
IEC is published by the International Electrical Commission and establishes standards for the basic safety and essential performance of medical electrical equipment The standards delineated in IEC have established a broadly accepted benchmark for medical electrical equipment. The table below lists all of the IEC X standards for particular types of medical equipment These standards amend the clauses of the basic standard You can purchase a standard from by clicking the standard you want. IEC is published by the International Electrical Commission and establishes standards for the basic safety and essential performance of medical electrical equipment The standards delineated in IEC have established a broadly accepted benchmark for medical electrical equipment.
Amendment 2 to IEC only clarifies how the operating voltage (decisive for planning the creepage distance) is to be determined To measure the operating voltage, all circuits must be earthed Isolated secondary circuits, which are isolated from earth by means of 1 MOP, are an exception. IEC is a series of technical standards for the safety and effectiveness of medical electrical equipment, published by the International Electrotechnical Commission First published in 1977 and regularly updated and restructured, as of it consists of a general standard, about 10 collateral standards, and about 80 particular standards. IEC , 32 Edition, August Medical electrical equipment – Part 1 General requirements for basic safety and essential performance This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of MEDICAL ELECTRICAL EQUIPMENT and MEDICAL ELECTRICAL SYSTEMS, hereafter referred to as ME EQUIPMENT and ME SYSTEMS.
19 of IEC has adopted the means of operator protection (MOOP) from IEC The reason is that for operators of medical devices no higher level of protection is required than that for laptop users The requirements are harmonised between the two standards as IT equipment is often used in ME systems. IEC Medical equipment must meet the design requirements as set out by the IEC (a harmonized standard), which has been adopted by all IEC member states This sets out all the design criteria for producing equipment that is electrically and mechanically safe, as well as placing the onus on the manufacturer to understand how to reduce the risk of harm when patients and operators are exposed to their medical devices. The Amendments Project under SC62A covers the general standard (IEC ) and most of the collateral standards (IEC XX, except for IEC ) (For background on the Amendments Project, refer to my previous article, “The Future of the IEC Series An Update,” published in the In Compliance Annual Reference Guide).
IEC , 41 Edition, September Medical electrical equipment – Part 12 General requirements for basic safety and essential performance – Collateral Standard Electromagnetic disturbances – Requirements and tests This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of MEDICAL ELECTRICAL EQUIPMENT and MEDICAL ELECTRICAL SYSTEMS, hereafter referred to as ME EQUIPMENT and ME SYSTEMS. Full Description IEC A112 contains requirements concerning basic safety and essential performance that are generally applicable to medical electrical equipment For certain types of medical electrical equipment, these requirements are either supplemented or modified by the special requirements of a collateral or particular standard. IEC/EN has wording that addresses the use of radios in a medical device An exemption for the main transmit signal from the radiated emissions limits (provided that they meet the national requirements) is given, but all other emissions must meet the radiated emissions limits of IEC/EN.
IEC is a series of technical standards that ensure the safety of medical electrical equipment. To answer these questions, we must review and understand the scope (subclause 11) of IEC to determine if and how the IEC Standard applies to a medical electrical product The title of IEC (3 rd edition) ( http//bitly/IEC ) is Medical electrical equipment – Part 1 General requirements for basic safety and essential performance. Intertek provides an engineering review of product design and development processes in line with the IEC standard on environmentally conscious design to help customers make important sustainability claims Learn the regulatory requirements for IEC , impact on certification schemes, including the IECEE CB scheme;.
Today we have the pleasure to announce that the cybersecurity technical report IEC TR has been published The not so short title of this report is Medical electrical equipment – Part 45 Guidance and interpretation – Safety related technical security specifications for medical devices. Regarding active medical devices, these requirements are documented and internationally harmonized under the standard family IEC Currently, the IEC 3rd edition is the base for the approval procedure of medical electrical equipment in most regulatory frameworks all over the world Due to identical requirements of the European Union’s EN family of standards, the IEC defines the assumption of conformity to Medical Device Directive (MDD) 93/42/EEC. IEC is a series of technical standards for the safety and effectiveness of medical electrical equipment, published by the International Electrotechnical Commission First published in 1977 and regularly updated and restructured, as of it consists of a general standard, about 10 collateral standards, and about 80 particular standards.
Iec 시리즈는 국제전기기술위원회에서 발행한 의료용 전기 장비의 안전과 필수 성능을 위한 일련의 기술 표준이다 1977년에 처음 발간되고 정기적으로 업데이트되고 재구성된 11년 현재 일반 표준, 약 10개의 보조 규격 및 약 60개의 개별 규격으로 구성되어 있다. The main IEC standard (referred to in Europe as EN and in Canada as CSA ) is an umbrella for numerous subsidiary standards, variously known as “collateral” or “particular” standards The 4 th edition is strictly one of these collateral standards known as, IEC “Electromagnetic disturbances Requirements and tests,” that has been extensively revised. IEC The International Electrotechnical Commission (IEC) is a worldwide body that promotes international standardization in electronics In 1993 it released the standard, "Medical Electrical Equipment—Part 1 General Requirements for Safety, Amendment No 2.
IEC applies to the basic safety and essential performance of medical electrical equipment and medical electrical systems for use in the home healthcare environment It applies regardless of whether the medical electrical equipment or medical electrical system is intended for use by a lay operator or by trained healthcare personnel. IEC , 41 Edition, September Medical electrical equipment – Part 12 General requirements for basic safety and essential performance – Collateral Standard Electromagnetic disturbances – Requirements and tests This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of MEDICAL ELECTRICAL EQUIPMENT and MEDICAL ELECTRICAL SYSTEMS, hereafter. To answer these questions, we must review and understand the scope (subclause 11) of IEC to determine if and how the IEC Standard applies to a medical electrical product The title of IEC (3 rd edition) ( http//bitly/IEC ) is Medical electrical equipment – Part 1 General requirements for basic safety and essential performance.
IEC applies to all electrical and electronic medical devices and their accessories The 3rd edition is in the process of being adopted by global regulatory authorities But on a national level, regulatory affectivity dates are not harmonized across global jurisdictions Parallel use of 2nd and 3rd edition is expected through 12. EN or IEC is the European harmonized standard to meet the Medical Device Directive EN applies to the basic safety and essential performance of medical electrical equipment and medical electrical systems. High frequency surgical equipment;.
EN is a group of standards which cover the safety, essential performance and electromagnetic compatibility of medical electrical equipment and related systems It is equivalent to the international standard IEC and comprises over 70 individual standards. Amendment 2 to IEC only clarifies how the operating voltage (decisive for planning the creepage distance) is to be determined To measure the operating voltage, all circuits must be earthed Isolated secondary circuits, which are isolated from earth by means of 1 MOP, are an exception. IEC pdf free downloadMedical electrical equipment – Part 110 General requirements for basic safety and essential performance – Collateral Standard Requirements for the development of physiologic closedloop controllers.
The International Medical Device EMC Standard—IEC June 10, 08 Daniel D Hoolihan Articles, Cable & Connectors, Lightning & Surge, Markets, Medical, Standards, Technologies, Testing The “2” standard is increasingly important in the electrical medical device world Dan Hoolihan, Houlihan EMC Consulting, Lindstrom, MN, USA.

Iec Medical Electrical Equipment Classification Faqs Medical Device Academy Medical Device Academy
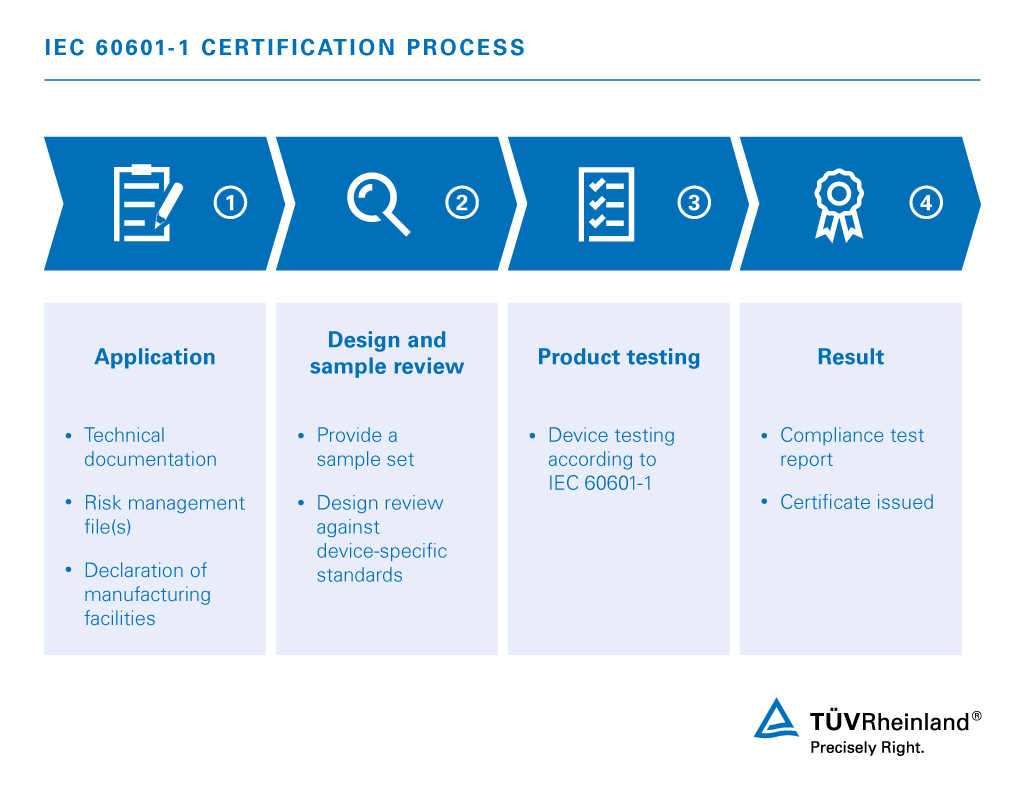
Iec 1 Medical Electrical Equipment Id Tuv Rheinland
Q Tbn And9gctbwh21hecrplnpndfboc9gkt Q4wlopklaozd8xlj3uk0htvqe Usqp Cau
Iec 60601 のギャラリー
Http Www Intertek Com Uploadedfiles Intertek Divisions Commercial And Electrical Media Pdf Medical Equipment Major Iec 1 3rd Ed Changes 9 14 10 Pdf
Http Pubweb2 Iec Ch Emc Emc News Pdf 16 6 Apemc 16 Medical Electronics Rsi V01 Pdf

Iec 1 Patient Applied Parts For Medical Electrical Equipment Medical Device Academy
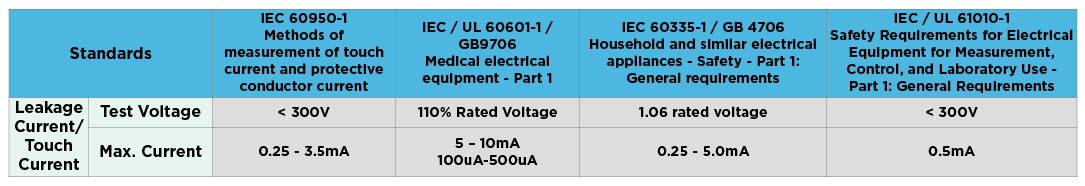
Extech Explores Eec Safety Testers Completes Iec Medical Testing Eec Extech Electronics Co Ltd

Home Healthcare Iec 1 11
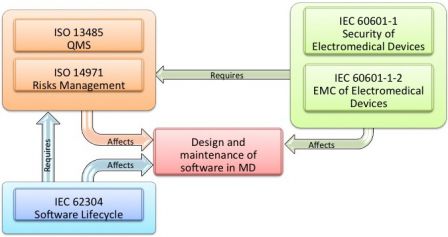
Md And Ivd Standards Iec 1 And Iec 1 Versus Iec Part 1 Software In Medical Devices By Md101 Consulting
Interpretation Of Iec 1 2 Electromagnetic Compatibility Requirements Tests

Lcb 4

Review Of Iec 1 2 14 4th Edition Interference Technology

Iec Archives Medical Device Academy Medical Device Academy

Event Singapore Manufacturing Federation

Iec 1 2 4th Edition New Darryl Ray Emc Consulting Llc

Medical Emc Requirements In Iec 1 2 07 14 Emc Standards

Implementing The Iec 1 Medical Electrical Equipment Standard Mddionline Com

How To Stay Up To Date On The Ever Changing Landscape Of The Medical Electrical Device Regulatory World In Compliance Magazine
Iec Medical Design Standards For Power Supplies Plianced Inc

Iec 1 X Medical Electrical Equipment State 08 Certifico Srl

Overview Of Iec 1 Terminology Definitions Through Annexes
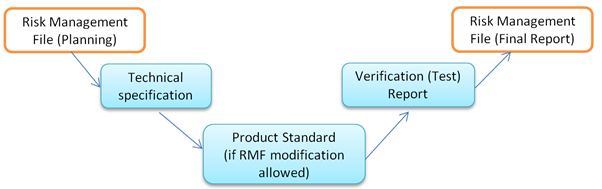
Iec 1 Clause 4 2 Risk Management Medteq
Q Tbn And9gcsgilofazndfvq0jsea6ck4c T D1vkbwa2avlonkg Usqp Cau

Workshop Design And Control On Iec 1 General Electrical Ul Asean

Iec 1 Amendment 2 Just Released Join For A Webinar On Changes More Eisner Safety Consultants

Iec 1 Ed 3 0 B 05

15 Steps To Get Approval To Iec 1

Microtips Technology Introduces Iec Certified Medical Monitors

Iec 1 Medical Testing Electrical Safety Testing Lab

Iec 1 Medical Power Safety Requirement

Download Your Free Guide To Iec Today Rigel Medical
Q Tbn And9gcrqzgp65vptzc Iemtn6kbvgahireakh5r2uk7dpm4 Usqp Cau

Iec 1 2 Ed 2 1 B 05 Medical Electrical Equipment Part 1 2 General Requirements For Safety Collateral Standard Electromagnetic Compatibility Requirements And Tests Iec Tc Sc 62a Amazon Com Books
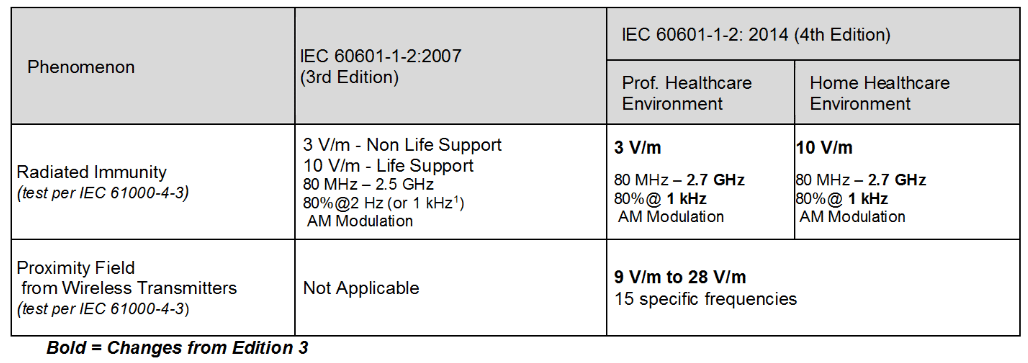
Review Of Iec 1 2 14 4th Edition Interference Technology

Contours Of The Eight Iec Alarms A Notation B And Download Scientific Diagram

What You Need To Know About Medical Electrical Standards Updates And
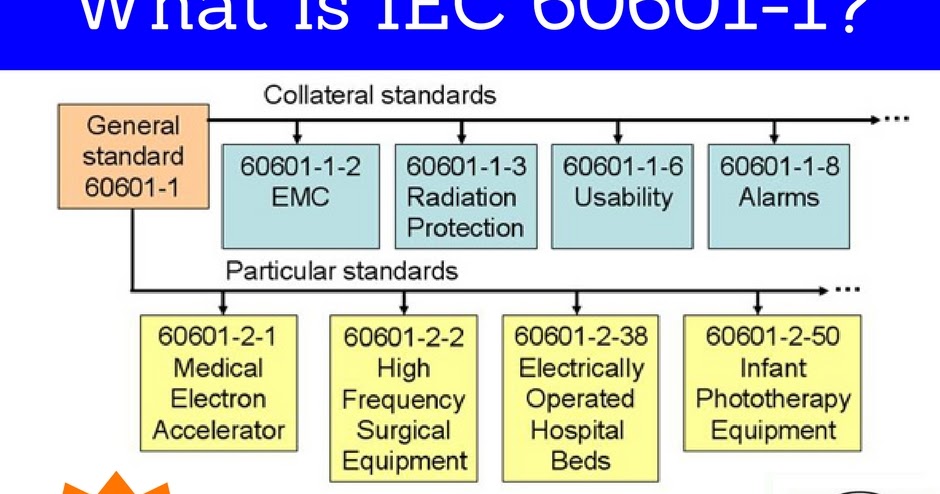
Electrical Safety Testing Lab Itc India What Is Iec 1
Iec 1 Sip Sio Isolation And Touch Current Requirements

Medical Emc Iec 1 2 14 Edition 4 Northwest Emc

Iec 1 12 14 Amd1 Amendment 1 Medical Electrical Equipment Part 1 12 General Requirements For Basic Safety And Essential Performance Collateral Standard Requirements For Medical Electrical Equipment And Medical Electrical Systems

National Deviations To Iec 1 Mddionline Com

Iec 1 Amd 1 Ed 3 0 B 12 Amendment 1 Medical Electrical Equipment Part 1 General Requirements For Basic Safety And Essential Performance

What Is The Iec Scope Medical Device Academy Medical Device Academy
Ewh Ieee Org R6 Ocs Pses Iec 1 The New Philosophy Of The 3rd Edition Pdf

Iec 1 Medical Power Supplies Cui Inc
3

Benefits Of Using Iec Compliant Components In Medical Devices
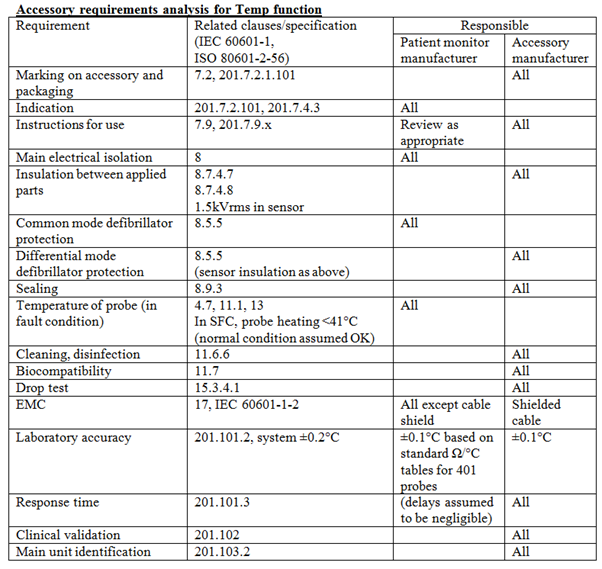
Iec 1 And Accessories Medteq

En Iec Testing Labs Testing Laboratories In India Led Testing Equipments Led Testing And Measurement Instruments

High And Low Priority Temporal Patterns Specified In Iec 1 8 The Download Scientific Diagram

Guide A Practical Guide To The Iec Standard Rigel Medical

What You Need To Know About Medical Electrical Standards Updates And
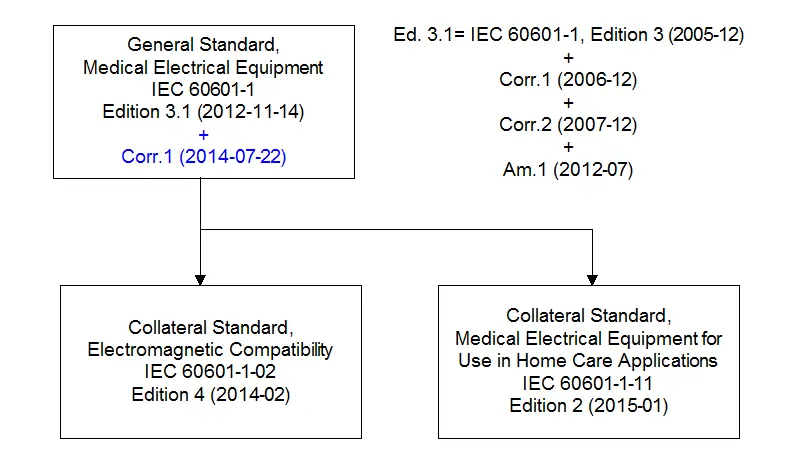
1 Edition 3 1 And Understanding Iec 1 Document Structure Globtek

15 Steps To Getting Approval For Iec 1

Ee Overview Of Iec 1 Scope And Normative References

Iec 1 2 Medical Devices Top 16 Faqs

Extech Explores Eec Safety Testers Completes Iec Medical Testing Eec Extech Electronics Co Ltd

Iec 1 2 Ed 4 1 The Changes Emc Technologies

15 Steps To Getting Approval For Iec 1

Iec 1 12 14 Iec Standards Vde Publishing House

Iec 1 4 Ed 1 1 B 00

Iec 1 8 Ed 1 0 B 05 Medical Electrical Equipment Part 1 8 General Requirements For Safety Collateral Standard General Requirements Equipment And Medical Electrical Systems Iec Tc Sc 62a Amazon Com Books
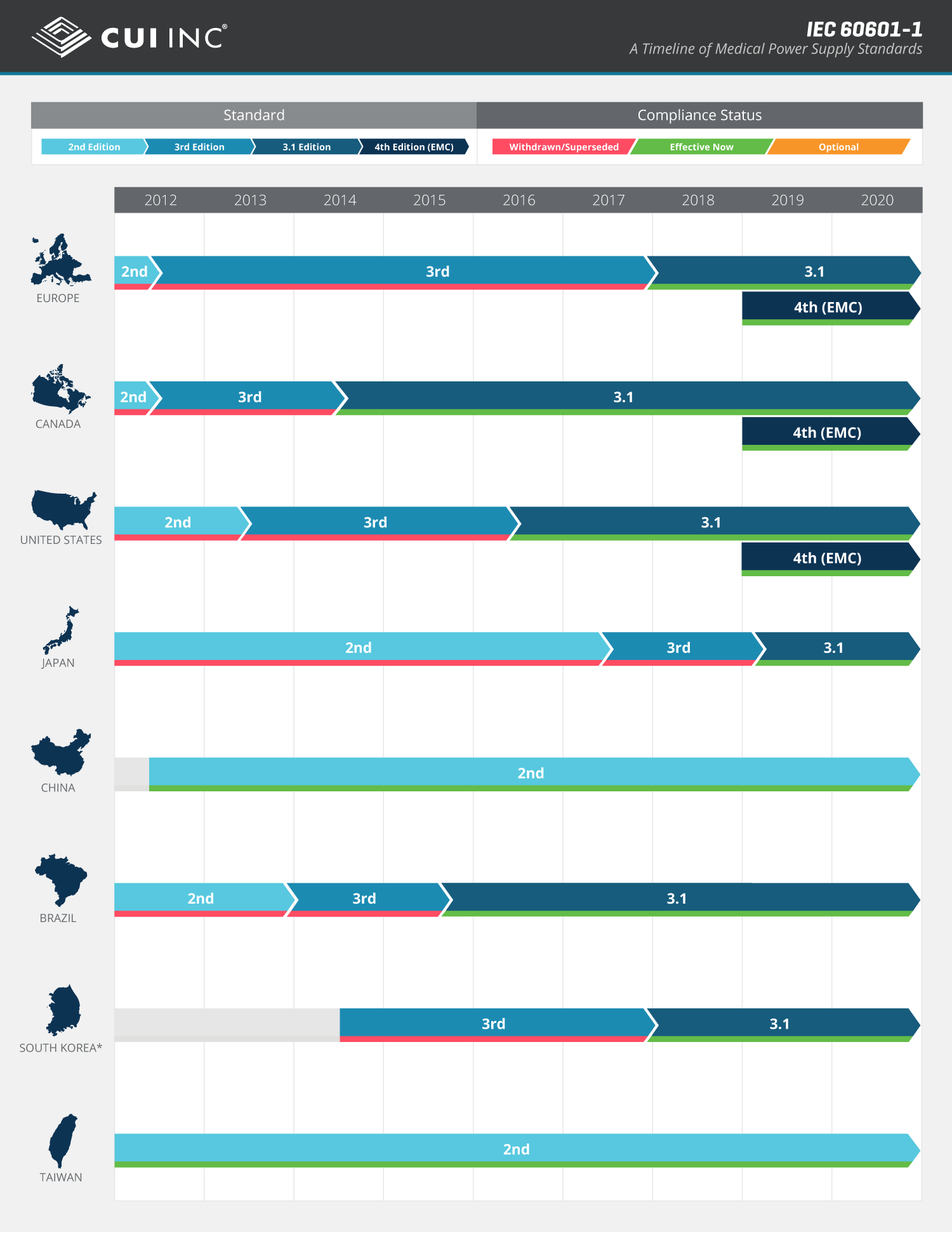
The Adoption Of Iec 1 Around The Globe Infographic

Overview Of Iec 1 Terminology Definitions Through Annexes

Iec 1 Edition 3 1 Introduces New Product Safety Requirements For Medical Electrical Equipment Eurofins E E North America

Amazon In Buy Iec 2 22 Ed 2 0 B 1995 Medical Electrical Equipment Part 2 Particular Requirements For The Safety Of Diagnostic And Therapeutic Laser Equipment Book Online At Low Prices In India
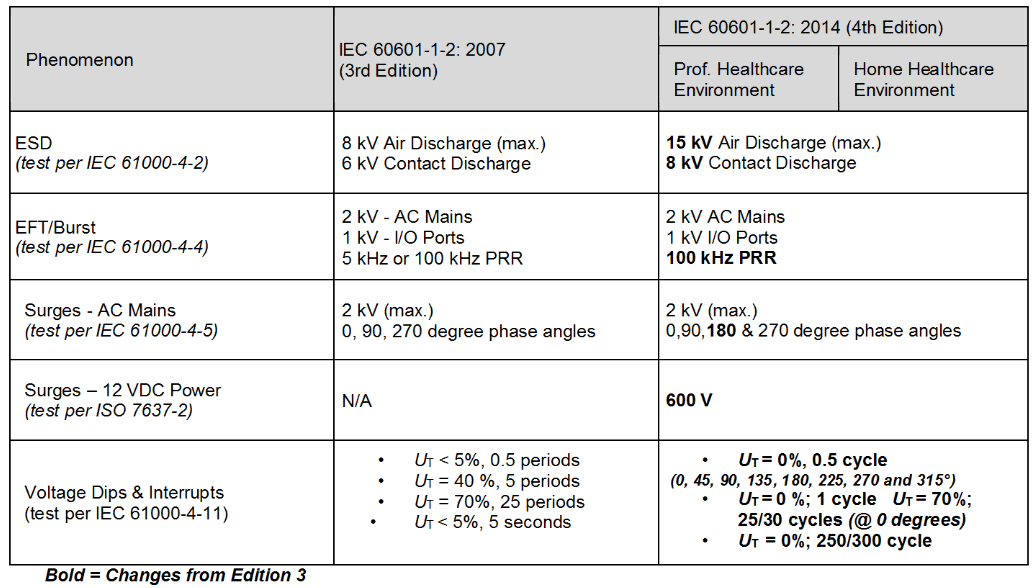
Review Of Iec 1 2 14 4th Edition Interference Technology
Iec 4 5 The Standard For It Security Is It Also For Stand Alone Software

Steps To Iec 1 Approval Compliance4all
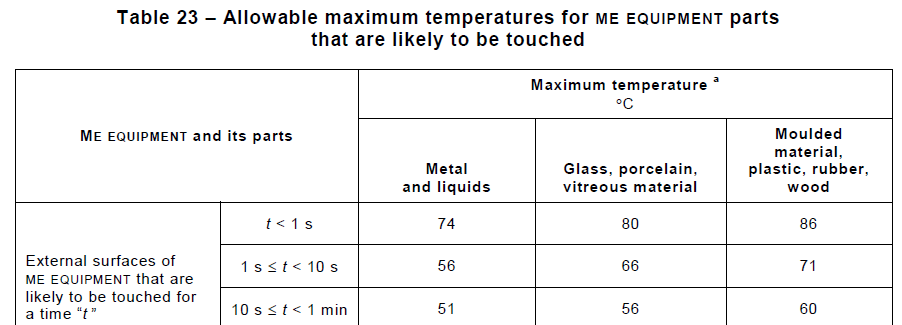
Overview Of Iec 1 Terminology Definitions Through Annexes
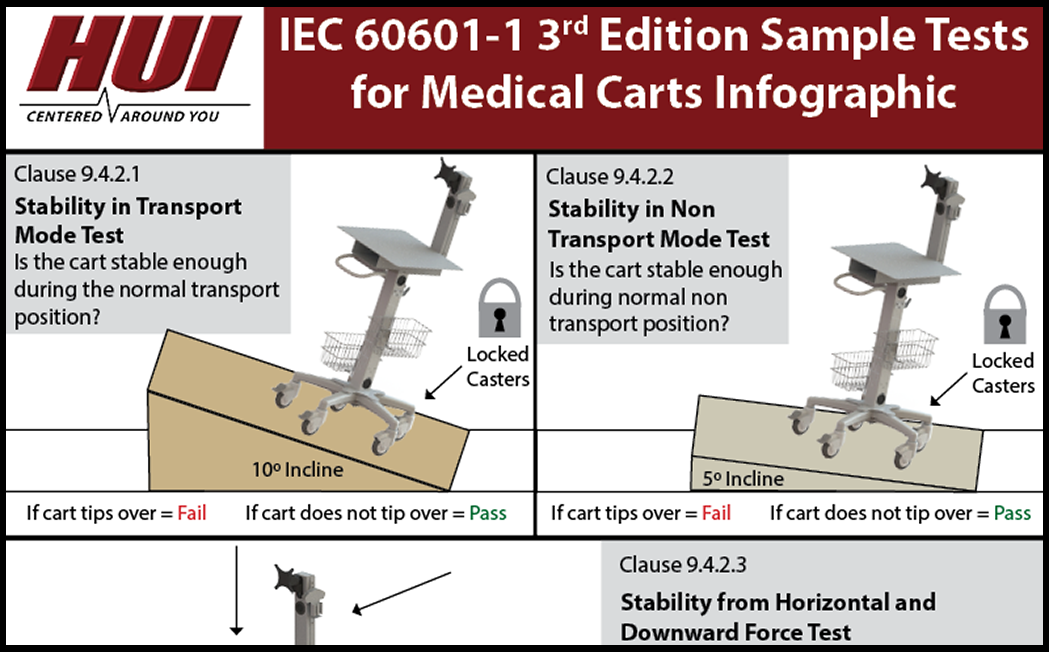
Infographic Iec 1 3rd Edition Sample Tests For Medical Carts
Http Ptitest Com Wp Content Uploads 13 02 Iec Family Of Standards 2 13 Presentation Pdf

Iec 1 Medical Power Supplies Cui Inc

New Iec Collateral Standards Published Early Eisner Safety Consultants

Online Iec Compliance Services Ideal Quality Certifications Id

Usability Standard Iec 1 6 In The Medical Device Industry

Ee Overview Of Iec 1 Scope And Normative References

Elexes Blog Iec 1 Evolution Of Electrical Safety

Main Results In Iec 2 25 Tests Download Table

Iec Explained By Leo Eisner Medical Devices Youtube

Elexes Blog Iec 1 Evolution Of Electrical Safety
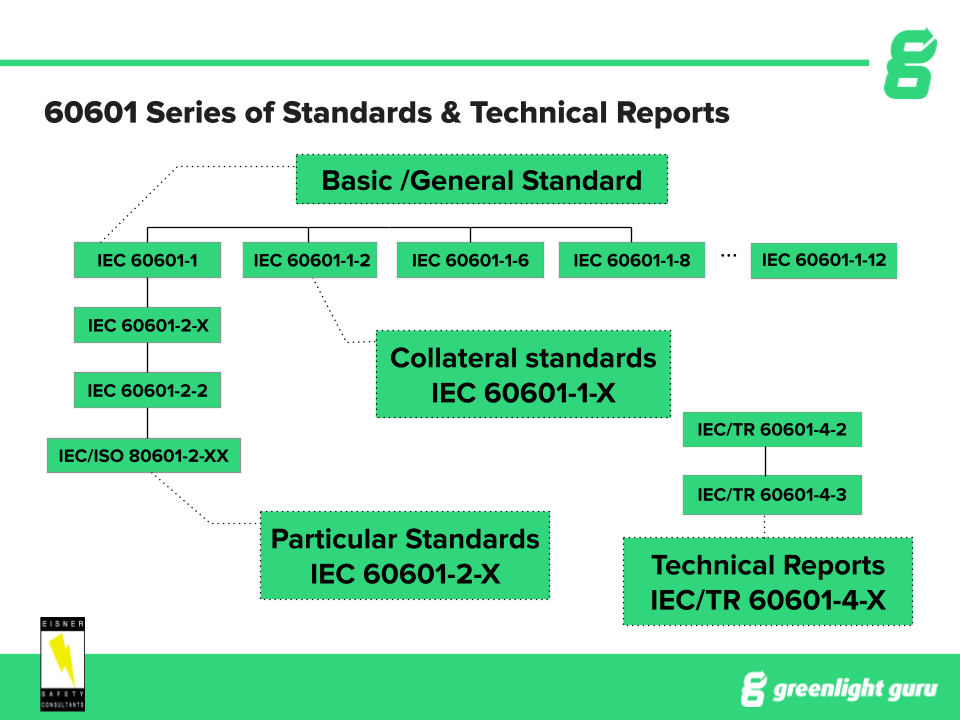
15 Steps To Getting Approval For Iec 1

Iec 1 2 4th Edition Are You Ready The Realtime Group
Www Nwemc Com Sites Www Nwemc Com Files Iec 1 2 4th Ed brodie pedersen Pdf

Overview Of Iec 1 Terminology Definitions Through Annexes

Elexes Blog Iec 1 Evolution Of Electrical Safety

Introduction To Iec What Medtech Developers Need To Know
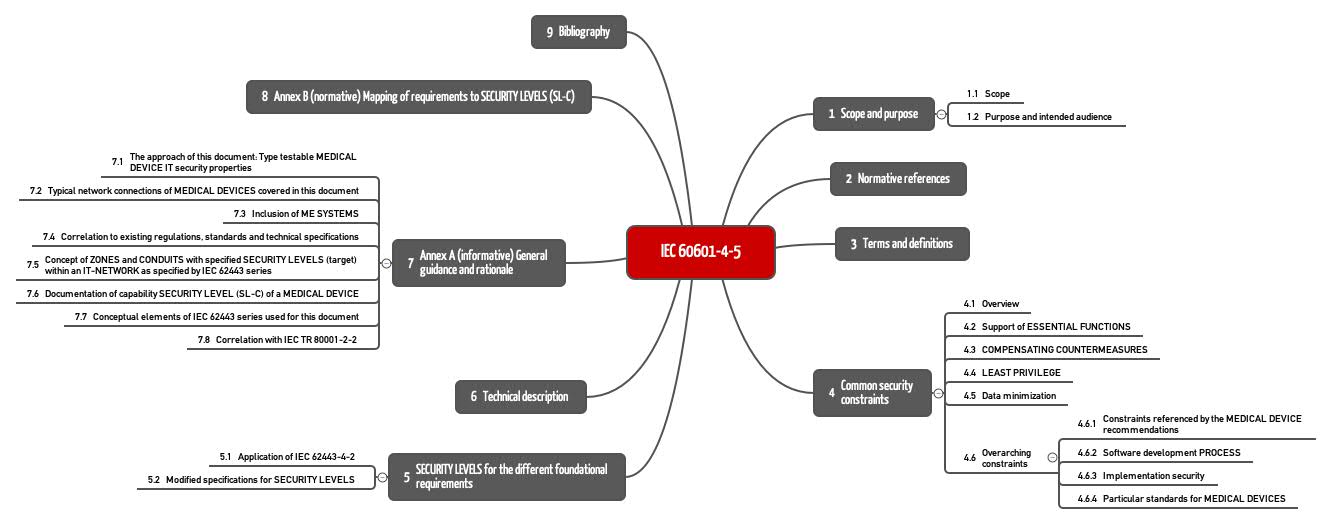
Iec 4 5 The Standard For It Security Is It Also For Stand Alone Software
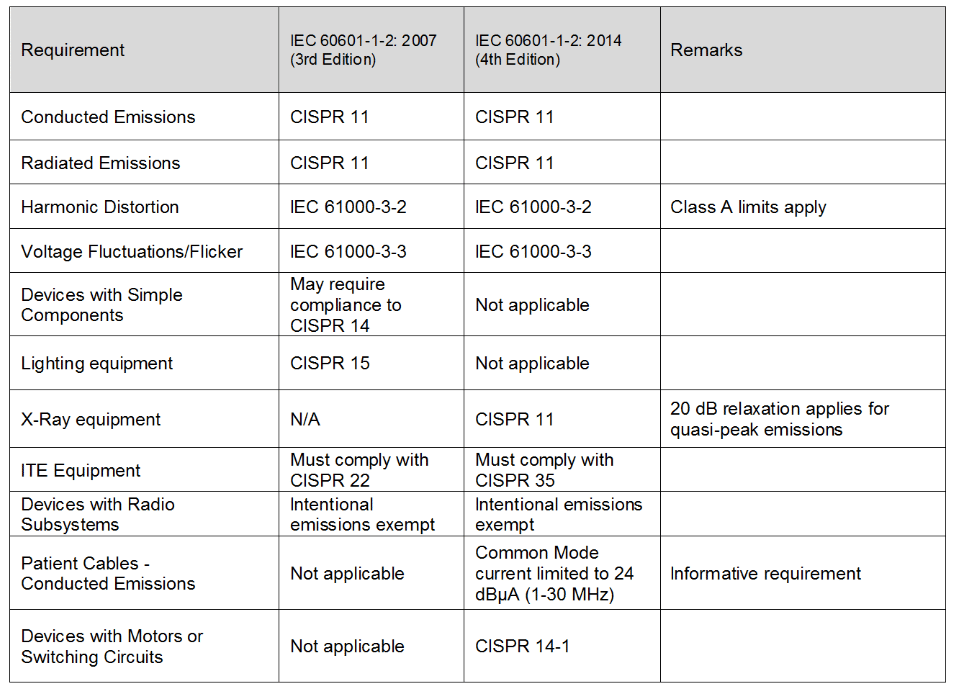
Review Of Iec 1 2 14 4th Edition Interference Technology

A Guide To Meeting The Iec 1 11 Standard In Home Healthcare Devices Medical Design And Outsourcing

Iec Just Got Amended Regulatory
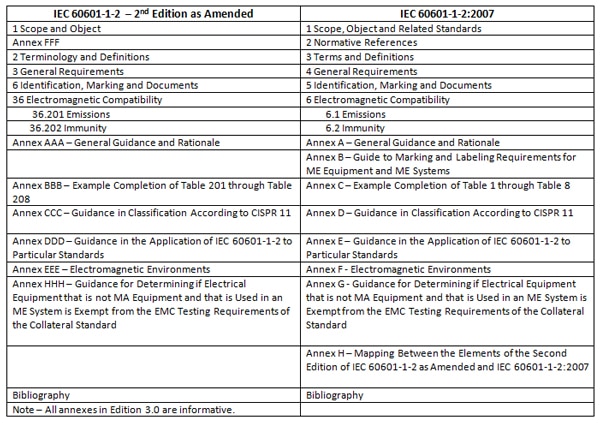
The International Medical Device Emc Standard Iec 1 2 Interference Technology

Iec 1 2 4th Edition
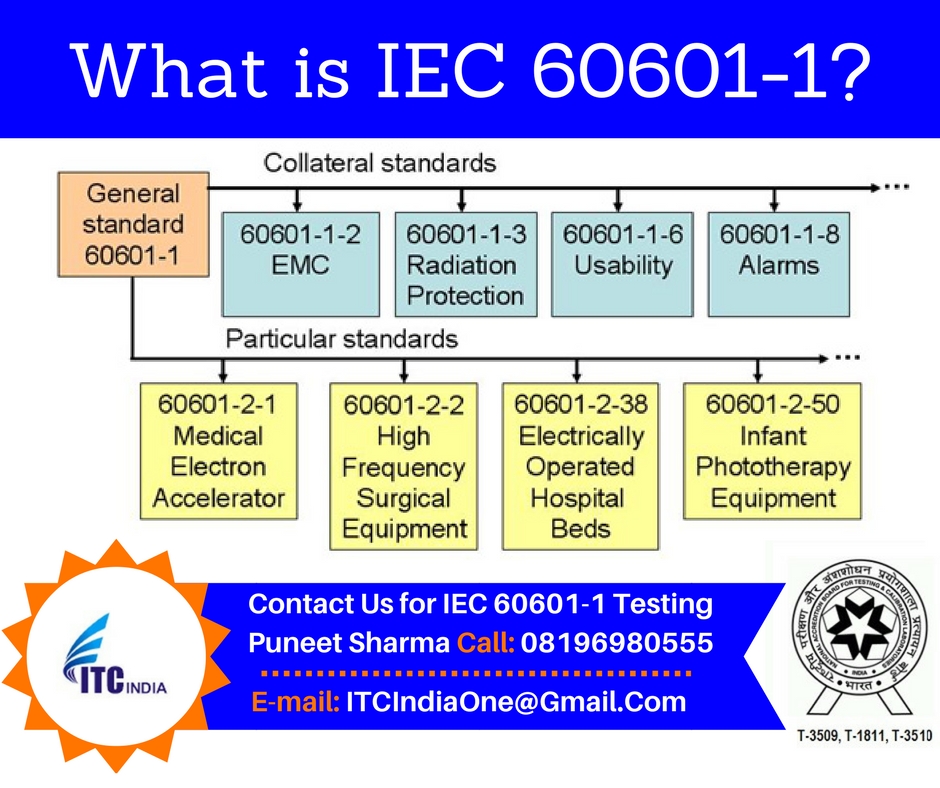
Electrical Safety Testing Lab Itc India What Is Iec 1
Iec 1 2 4th Edition What You Need To Know Cui Inc

Electrical Safety For Active Medical Devices The Iec 1 Standard Vde Medical Devices And Software

Iec 1 Ed 3 2 En

Iec Amendments For Medical Electrical Equipment Medical Device Academy

Hypothetical Design Guidance Making A Simple Medical Device Iec 1 Electrically Compliant Ece

New Medical Device Emc Requirements Medical Design Briefs

Emc Requirements Pending Changes For The Fourth Edition Of Iec 1 2 Medical Design Briefs

Cui Inc Global Adoption Of Iec 1 Arrow Com
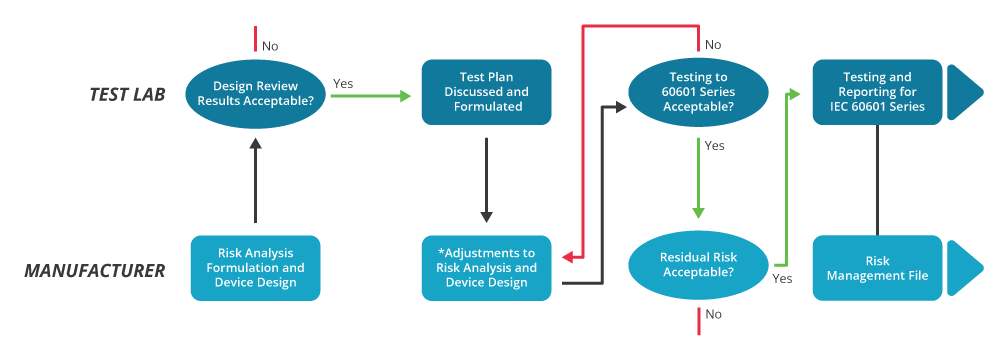
Iec 1 Medical Design Standards For Power Supplies Cui Inc

Overview Of 1 3rd Edition Webinar Youtube



